
TEBETANE COMPOUND Capsules

How to use TEBETANE COMPOUND Capsules
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Tavulus 18 micrograms powder for inhalation (hard capsule)
Tiotropium
Read this package leaflet carefully before you start using this medicine, because it contains important information for you.
- Keep this package leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this package leaflet. See section 4.
Contents of the package leaflet:
- What is Tavulus 18 micrograms and what is it used for
- What you need to know before you start using Tavulus 18 micrograms
- How to use Tavulus 18 micrograms
- Possible side effects
- Storage of Tavulus 18 micrograms
- Contents of the pack and further information
1. What is Tavulus 18 micrograms and what is it used for
This medicine contains the active substance tiotropium. This medicine helps people with chronic obstructive pulmonary disease (COPD) to breathe more easily. Chronic obstructive pulmonary disease (COPD) is a long-term lung disease that makes it difficult to breathe and causes coughing. The term COPD is associated with chronic bronchitis and emphysema. Since COPD is a chronic disease, you must use this medicine every day and not just when you have breathing problems or other symptoms of COPD.
This medicine is a long-acting bronchodilator that helps to open up the airways and makes it easier to take and expel air from the lungs. Regular use of this medicine can also help you when you have difficulty breathing continuously due to your disease, and it will help you minimize the effects of the disease on your daily life. It also helps you to be active for longer. Daily use of this medicine will also help you prevent sudden and short-term symptoms of worsening COPD, which can last for several days. The effect of this medicine lasts for 24 hours, so you only need to use it once a day. For the correct dosing of this medicine, see section 3. How to use Tavulus 18 micrograms and the instructions for use at the end of this package leaflet.
2. What you need to know before you start using Tavulus 18 micrograms
Do not use Tavulus 18 micrograms
- If you are allergic (hypersensitive) to tiotropium or any of the other ingredients of this medicine (listed in section 6).
- If you are allergic (hypersensitive) to atropine or its derivatives, such as ipratropium or oxitropium.
Warnings and precautions
Talk to your doctor or pharmacist before you start using this medicine:
- Tell your doctor if you have narrow-angle glaucoma, prostate problems, or difficulty urinating.
- If you have kidney problems, please talk to your doctor.
- This medicine is intended for the maintenance treatment of your chronic obstructive pulmonary disease, it must not be used to treat a sudden episode of shortness of breath or wheezing.
- After administration of this medicine, immediate allergic reactions such as rash, swelling, itching, wheezing, or shortness of breath may occur. If this happens, please talk to your doctor immediately.
- Inhaled medicines like this medicine may cause chest tightness, coughing, wheezing, or shortness of breath immediately after inhalation. If this happens, please talk to your doctor immediately.
- Be careful not to get the powder into your eyes, as this may cause or worsen narrow-angle glaucoma, which is an eye disease. Eye pain or discomfort, blurred vision, halos around lights, or colored images associated with redness of the eyes may be signs of an acute attack of narrow-angle glaucoma. Eye symptoms may be accompanied by headache, nausea, or vomiting. You should stop using tiotropium bromide and talk to your doctor immediately, preferably an eye doctor, when you experience signs and symptoms of narrow-angle glaucoma.
- Dry mouth, which has been observed during treatment with anticholinergics, may be associated with dental caries in the long term. Therefore, remember to take care of your oral hygiene.
- If you have had a heart attack in the last 6 months or unstable irregular heartbeats, or life-threatening heart failure in the last year, tell your doctor. This is important to decide if this medicine is the right medicine for you.
- You must not use this medicine more than once a day.
Children and adolescents
This medicine is not recommended for children and adolescents under 18 years of age.
Other medicines and Tavulus 18 micrograms
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
Tell your doctor or pharmacist if you are using or have used similar medicines for your lung disease, such as ipratropium or oxitropium.
No specific adverse reactions have been reported when this medicine has been used together with other medicines commonly used for the treatment of COPD, such as rescue inhalers, e.g., salbutamol, methylxanthines, such as theophylline, and/or oral and inhaled steroids, such as prednisolone.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine. You must not use this medicine unless your doctor has specifically recommended it.
Driving and using machines
Dizziness, blurred vision, or headache may affect your ability to drive or use machines.
Tavulus 18 micrograms contains lactose monohydrate
If you are administered according to the recommended dose, one capsule once a day, each dose provides up to 5.5 mg of lactose monohydrate. If your doctor has told you that you have an intolerance to some sugars, talk to them before taking this medicine.
3. How to use Tavulus 18 micrograms
Follow the instructions for administration of this medicine exactly as told by your doctor or pharmacist. If you are in doubt, talk to your doctor or pharmacist again.
The recommended dose is the inhalation of the contents of one capsule (18 micrograms of tiotropium) once a day. Do not use more than the recommended dose.
This medicine is not recommended for children and adolescents under 18 years of age.
You should try to use the capsule at the same time every day. This is important because this medicine is effective for 24 hours.
The capsules are for inhalation only and not for oral ingestion. Do not swallow the capsules.
The dry powder inhaler (MRX003-T10), into which you must insert the capsule of this medicine, pierces the capsule and allows you to inhale the powder.
Make sure you have a dry powder inhaler and that you are able to use it correctly. The instructions for use of the dry powder inhaler are at the end of this package leaflet.
Make sure you do not blow into the dry powder inhaler.
If you have any problems using the dry powder inhaler, ask your doctor, nurse, or pharmacist to show you how it works.
You must clean your dry powder inhaler once a month. The instructions for cleaning the dry powder inhaler are at the end of this package leaflet.
When you use this medicine, be careful and do not let the powder get into your eyes. If the powder gets into your eyes, it could cause blurred vision, pain, and/or eye redness. You must rinse your eyes immediatelywith warm water. Talk to your doctor immediatelyfor more information.
If you notice that your breathing gets worse, talk to your doctor as soon as possible.
If you use more Tavulus 18 micrograms than you should
If you inhale more than 1 capsule of this medicine in one day, you must talk to your doctor immediately. You may have a higher risk of experiencing a side effect such as dry mouth, constipation, difficulty urinating, increased heart rate, or blurred vision.
In case of overdose or accidental ingestion, contact the Toxicological Information Service. Phone: 91 562 04 20, talk to your doctor or pharmacist.
If you forget to use Tavulus 18 micrograms
If you have forgotten a dose, take a dose as soon as you remember, but do nottake two doses at the same time or on the same day. Then take your next dose as usual. Do not take a double dose to make up for forgotten doses.
If you stop using Tavulus 18 micrograms
Before you stop using this medicine, you must talk to your doctor or pharmacist. If you stop using this medicine, the signs and symptoms of your COPD may get worse.
If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. The side effects described below have been experienced by people taking this medicine.
After administration of this medicine, individual or severe allergic reactions (anaphylactic reaction) may occur, including allergic reactions that cause swelling of the face or throat (angioedema) or other hypersensitivity reactions (such as a sudden drop in blood pressure or dizziness). Additionally, as with all inhaled medicines, some patients may experience unexpected chest tightness, coughing, wheezing, or shortness of breath immediately after inhalation (bronchospasm). If you experience any of these side effects, talk to your doctor immediately.
Common (may affect up to 1 in 10 people):
- Dry mouth: this is usually mild
Uncommon (may affect up to 1 in 100 people):
- dizziness
- headache
- changes in taste
- blurred vision
- irregular heart rhythm (atrial fibrillation)
- throat inflammation (pharyngitis)
- hoarseness (dysphonia)
- coughing
- heartburn (gastroesophageal reflux disease)
- constipation
- fungal infection in the mouth or throat (oropharyngeal candidiasis)
- rash
- difficulty urinating (urinary retention)
- painful urination (dysuria)
Rare (may affect up to 1 in 1,000 people):
- difficulty sleeping (insomnia)
- halos around lights or colored images associated with redness of the eyes (glaucoma)
- increased eye pressure
- irregular heart rhythm (supraventricular tachycardia)
- increased heart rate (tachycardia)
- feeling your heart beating (palpitations)
- chest tightness associated with coughing, wheezing, or shortness of breath immediately after inhalation (bronchospasm)
- nosebleed (epistaxis)
- larynx inflammation (laryngitis)
- sinus inflammation (sinusitis)
- intestinal blockage or absence of bowel movement (intestinal obstruction including paralytic ileus)
- gum inflammation (gingivitis)
- tongue inflammation (glossitis)
- difficulty swallowing (dysphagia)
- mouth inflammation (stomatitis)
- feeling sick (nausea)
- allergic reactions (hypersensitivity), including immediate reactions
- severe allergic reaction that causes swelling of the face and throat (angioedema)
- skin rash (urticaria)
- itching (pruritus)
- urinary tract infection
Not known (cannot be estimated from the available data):
- loss of body water (dehydration)
- tooth decay
- severe allergic reaction (anaphylactic reaction)
- skin infections or ulcers
- dry skin
- joint swelling
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this package leaflet. You can also report side effects directly through the Spanish Pharmacovigilance System for Human Use Medicines: http://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Tavulus 18 micrograms
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the packaging and on the blister. The expiry date is the last day of the month stated.
Always use the capsule directly, within 30 minutes after opening the blister. Each dry powder inhaler must be replaced after 6 months, which corresponds to the administration of a maximum of 180 capsules.
Do not store above 30°C.
Keep the medicine in the original package to protect it from moisture.
Medicines should not be disposed of via wastewater or household waste. Return the containers and any unused medicines to the pharmacy. Ask your pharmacist how to dispose of medicines no longer required. This will help to protect the environment.
6. Container Contents and Additional Information
Composition of Tavulus 18 micrograms
- The active ingredient is tiotropium. Each capsule contains 18 micrograms of active ingredient tiotropium (as bromide). During inhalation, 10 micrograms of tiotropium are released from the mouthpiece of the dry powder inhaler.
- The other component is lactose monohydrate (which may contain small amounts of milk proteins).
Appearance of the Product and Container Contents
The Tavulus 18 microgram capsules, which release 10 micrograms of powder for inhalation per dose, are hard, colorless, and transparent capsules that contain a white powder and are marked with "T10" printed on the capsules.
This medication is presented in blisters. The containers contain the blisters and the dry powder inhaler. The dry powder inhaler has a white body with a red button.
Package formats:
- Carton box containing 30 capsules (3 blisters) with a dry powder inhaler
- Carton box containing 60 capsules (6 blisters) with a dry powder inhaler
- Carton box containing 90 capsules (9 blisters) with a dry powder inhaler
- Carton box containing 30 capsules (3 blisters)
- Carton box containing 60 capsules (6 blisters)
- Carton box containing 90 capsules (9 blisters)
Only some package sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder
Zentiva k.s.
U Kabelovny 130
Dolní Mecholupy
102 37 Prague 10
Czech Republic
Manufacturer:
Helm Pharmaceuticals GmbHNordkanalstrasse 28
20097 Hamburg
Germany
You can request more information about this medication by contacting the local representative of the marketing authorization holder: Zentiva Spain S.L.U.
Avenida de Europa, 19, Edificio 3, Planta 1.
28224 Pozuelo de Alarcón, Madrid
Spain
This medication is authorized in the EEA member states under the following names:
Spain | Tavulus 18 micrograms powder for inhalation (hard capsule) |
France | Tiogiva 18 micrograms powder for inhalation in capsule |
Netherlands | Tavulus 18 micrograms, inhalation powder in hard capsules |
Date of the last revision of this prospectus:March 2024
Detailed information about this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/.
Instructions for using the dry powder inhaler (MRX003R-T10)
Dear patient,
The dry powder inhaler (MRX003-T10) allows you to inhale the medication contained in the Tavulus capsule prescribed by your doctor for your respiratory problems.
To ensure the correct administration of the medication, you must be trained by your doctor or any other healthcare professional on how to use the inhaler.
The dry powder inhaler is specially designed for Tavulus capsules, so it should not be used for any other medication.
Your medication comes with its Tavulus capsules in blisters.
Use, when applicable, the new dry powder inhaler provided with your medication. Each dry powder inhaler should be replaced after 6 months, which corresponds to a maximum administration of 180 capsules. Do not usethe device if you observe any deterioration or if the capsule remains in the device.
Figure A | The parts of your dry powder inhaler are (see Figure A):
|
Taking your daily dose of medication requires 4 main steps Step 1.Opening your dry powder inhaler: | |
| To open the protective cap of the dry powder inhaler:
|
|
|
Step 2.Insert a capsule into the dry powder inhaler | |
|
|
|
|
Step 3.Pierce the capsule | |
|
|
Step 4.Taking your daily dose (2 inhalations from the same capsule) | |
|
|
| In your next breath, inhale your medication:
Important: Do notpress the piercing button again. Remember: To receive the complete dose of medication every day, you must inhale 2 times from the same capsule. Make sure to exhale completely each time before inhaling with your dry powder inhaler. Do not exhale into your dry powder inhaler. |
Care and maintenance of your dry powder inhaler: | |
| After taking your daily dose, open the mouthpiece and remove the capsule, disposing of it in the trash
Keep the dry powder inhaler at room temperature (not above 30°C). |
Cleaning your dry powder inhaler: | |
| Clean your dry powder inhaler monthly. It takes 24 hours for the dry powder inhaler to air dry after cleaning.
Steps for cleaning
|
Handling the blisters: | |
| Each day, separate only 1 of the blister cavities by tearing along the perforated line (see Figure K) |
| Removing the capsule from the blister:
|
| Each capsule contains only a small amount of powder (see Figure M). This is a complete dose Do not open the capsule, as it may not be usable. |
- Country of registration
- Average pharmacy price8.95 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to TEBETANE COMPOUND CapsulesDosage form: CAPSULE, 160 mgActive substance: Sabalis serrulatae fructusManufacturer: Pierre Fabre Iberica S.A.Prescription requiredDosage form: CAPSULE, 320 mgActive substance: Sabalis serrulatae fructusManufacturer: Bioforce Espana A Vogel S.A.Prescription not requiredDosage form: CAPSULE, 320 mgActive substance: Sabalis serrulatae fructusManufacturer: Laboratorios Q Pharma S.L.Prescription required
Online doctors for TEBETANE COMPOUND Capsules
Discuss questions about TEBETANE COMPOUND Capsules, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions





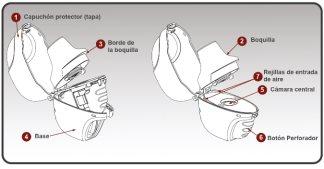
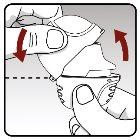 Figure B
Figure B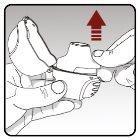 Figure C
Figure C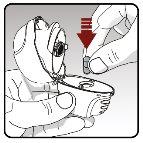 Figure D
Figure D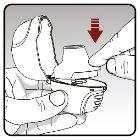 Figure E
Figure E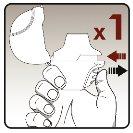 Figure F
Figure F Figure G
Figure G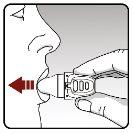 Figure H
Figure H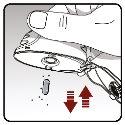 Figure I
Figure I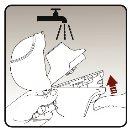 Figure J
Figure J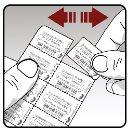 Figure K
Figure K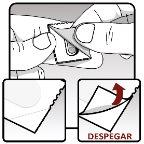 Figure L
Figure L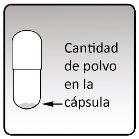 Figure M
Figure M
