
SYMBICORT 80 micrograms/2.25 micrograms/inhalation pressurized suspension for inhalation

How to use SYMBICORT 80 micrograms/2.25 micrograms/inhalation pressurized suspension for inhalation
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Symbicort 80micrograms/2.25micrograms/inhalation inhalation suspension in a pressurised inhaler
Budesonide/formoterol fumarate dihydrate
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What Symbicort is and what it is used for.
- What you need to know before you use Symbicort.
- How to use Symbicort.
- Possible side effects.
- Storing Symbicort.
- Contents of the pack and other information.
1. What Symbicort is and what it is used for
What Symbicort is
Symbicort is an inhaler used to treat asthma in adults and adolescents from 12 to 17 years of age. It contains two different medicines: budesonide and formoterol fumarate dihydrate.
- Budesonide belongs to a group of medicines called “corticosteroids”.
- Formoterol fumarate dihydrate belongs to a group of medicines called “long-acting beta2 adrenergic agonists” or “bronchodilators”.
What Symbicort is used for
Symbicort is used to treat asthma and may be prescribed alone or with another inhaler "for relief of symptoms" to be used separately:
Some patients are prescribed two inhalers for asthma: the Symbicort inhaler and another separate "reliever" inhaler
- They use Symbicort every day to help prevent asthma symptoms from occurring.
- They use their “reliever” inhaler when they have asthma symptoms to help them breathe.
Some patients are prescribed Symbicort as their only asthma inhaler
- They use Symbicort every day to help prevent asthma symptoms from occurring.
- They also use Symbicort when they need extra doses for relief of asthma symptoms, to help them breathe, and if agreed with their doctor, to prevent asthma symptoms from occurring (for example, before exercise or when exposed to other allergens). They do not need a separate inhaler for this purpose.
How Symbicort works
- Budesonide works by reducing and preventing inflammation in the lungs.
- Formoterol fumarate dihydrate works by relaxing the muscles in the airways, making it easier to breathe.
2. What you need to know before you use Symbicort
Do not use Symbicort:
- if you are allergic to budesonide, formoterol or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Consult your doctor or pharmacist before you start using Symbicort if:
- You have diabetes.
- You have a lung infection.
- You have high blood pressure or have ever had heart disease (including irregular heartbeats, fast pulse, narrowing of the arteries or heart failure).
- You have thyroid or adrenal gland problems.
- You have low levels of potassium in your blood.
- You have severe liver problems.
Consult your doctor if you experience blurred vision or other visual disturbances while using Symbicort.
Children
Do not use this medicine in children under 12 years of age.
Use in athletes
This medicine contains formoterol, which may produce a positive result in doping tests.
Other medicines and Symbicort
Tell your doctor or pharmacist if you are using, have recently used or might use any other medicines.
In particular, tell your doctor or pharmacist if you are using any of the following medicines:
- Beta-blocker medicines for high blood pressure, such as atenolol or propranolol.
- Eye drops, such as timolol for glaucoma.
- Medicines for an irregular heartbeat, such as quinidine.
- Medicines such as digoxin, which are usually used to treat heart failure.
- Diuretics for high blood pressure, such as furosemide.
- Oral steroid medicines, such as prednisolone.
- Xanthine medicines, such as theophylline or aminophylline, which are commonly used to treat asthma.
- Other bronchodilators, such as salbutamol.
- Antidepressants, such as amitriptyline and nefazodone.
- Phenothiazine medicines, such as chlorpromazine and prochlorperazine.
- Medicines for HIV infection, such as ritonavir.
- Medicines for infections, such as ketoconazole, itraconazole, voriconazole, posaconazole, clarithromycin and telithromycin.
- Medicines for Parkinson’s disease, such as levodopa.
- Medicines for thyroid problems, such as levothyroxine.
If you are in any of these situations, or if you are not sure, ask your doctor or pharmacist before using Symbicort.
Tell your doctor or pharmacist if you are going to have a general anaesthetic for surgery or for dental treatment.
Pregnancy and breastfeeding
- Tell your doctor before using this medicine if you are pregnant or planning to become pregnant; consult your doctor before using Symbicort - do not use Symbicort unless your doctor tells you to.
- If you become pregnant while using Symbicort, do not stop using it but consult your doctor immediately.
- If you are breastfeeding, consult your doctor before using Symbicort.
Driving and using machines
Symbicort has no or negligible influence on the ability to drive and use machines.
3. How to use Symbicort
Follow the administration instructions for this medication exactly as indicated by your doctor or pharmacist. If in doubt, consult your doctor or pharmacist again.
- It is essential to use Symbicort daily, even if you do not have symptoms of asthma at that time.
- Your doctor will want to regularly check how your asthma symptoms are going.
- Your doctor may consider adding steroid tablets to your usual treatment during periods of stress (e.g., when you have a chest infection or before an operation).
Symbicort may be prescribed for asthma alone or with another reliever inhaler separately. You should take two inhalations of Symbicort to get a full dose. The amount of Symbicort you should use and when you should use it will depend on what you have been prescribed.
- If you have been prescribed Symbicort and a separate reliever inhaler, read the section "a) Using Symbicort and another inhaler separately for relief of symptoms".
- If you have been prescribed Symbicort as your only inhaler, read the section "b) Using Symbicort as your only inhaler for asthma".
- Using Symbicort and another inhaler separately for relief of symptoms
Use Symbicort daily. This helps prevent asthma symptoms.
Adults (over 18 years)
- The usual dose is 2-4 inhalations, twice a day.
- Your doctor may prescribe up to a maximum of 8 inhalations, twice a day.
- If your asthma symptoms are well-controlled, your doctor may ask you to use the medication once a day.
Adolescents (12 to 17 years)
- The usual dose is 2-4 inhalations, twice a day.
- If your asthma symptoms are well-controlled, your doctor may ask you to use the medication once a day.
Your doctor (or nurse) will help you manage your asthma. They will adjust the dose of this medication to the lowest dose that controls your asthma. However, do not adjust or stop the dose without talking to your doctor (or nurse) first.
Use your other "reliever inhaler" when asthma symptoms appear.
- Always keep this "reliever inhaler" with you so you can use it when you need it.
- Do not use Symbicort to treat asthma symptoms; use your reliever inhaler instead.
- Using Symbicort as your only inhaler for asthma
Use Symbicort in this way only if your doctor has told you to and if you are over 12 years old.
Use Symbicort daily. This helps prevent asthma symptoms from appearing. You can do:
- 2 inhalations in the morning and 2 inhalations at night.
- 4 inhalations in the morning.
- 4 inhalations at night.
Your doctor may increase the dose to 4 inhalations, twice a day.
Also, use Symbicort as a "reliever inhaler" to treat asthma symptoms when they appear and to prevent asthma symptoms from appearing (e.g., when exercising or exposed to allergens).
- If you have asthma symptoms, take 2 inhalations and wait a few minutes.
- If you do not feel better, take another 2 inhalations.
- Do not take more than 12 inhalations at one time.
Always keep your Symbicort inhaler with you so you can use it when you need it.
Normally, a daily dose of more than 16 inhalations is not required. However, your doctor may allow you to take up to 24 inhalations per day for a limited period.
If you usually need to use 16 or more inhalations per day, go to your doctor or nurse, as you may need to change your treatment.
Do not take more than 24 inhalations in 24 hours.
If you are exercising and notice asthma symptoms, use Symbicort as described here. It is essential to talk to your doctor about using Symbicort to prevent asthma symptoms; both the frequency of exercise and exposure to allergens could influence the treatment prescribed to you.
Symbicort and steroid tablets
If you have been taking steroid tablets for asthma, your doctor may reduce the number of tablets you take once you start treatment with Symbicort. If you have been taking oral steroid tablets for a long time, your doctor may want to do a blood test from time to time. When the number of oral steroid tablets is reduced, you may feel unwell, even if your lung symptoms are improving. The symptoms can be:
- nasal congestion or runny nose
- weakness
- joint or muscle pain
- skin rash (hives).
Consult your doctor immediately if you experience symptoms such as:
- headache
- fatigue
- nausea
- vomiting.
If you experience any of the above symptoms, consult your doctor immediately; you may need to take another medication. Talk to your doctor if you are concerned about whether you should continue using Symbicort.
Use in children
There are other formulations of this medication suitable for children; consult your doctor or pharmacist.
Important information about your asthma symptoms
If, while using Symbicort, you feel short of breath or wheeze, you should continue using it and contact your doctor as soon as possible, as you may need additional treatment.
Consult your doctor immediately if:
- Your breathing is getting worse or you often wake up at night with asthma symptoms.
- You start to feel chest tightness in the morning, or the chest tightness lasts longer than usual.
- These signs may indicate that your asthma is not adequately controlled, and you may need a different or additional treatment immediately.
Information about your new Symbicort inhaler
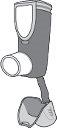 Before using your new Symbicort inhaler, remove it from the aluminum paper wrapper. Dispose of the wrapper and the desiccant inside the wrapper. Do not use the inhaler if the desiccant has come out of the package.
Before using your new Symbicort inhaler, remove it from the aluminum paper wrapper. Dispose of the wrapper and the desiccant inside the wrapper. Do not use the inhaler if the desiccant has come out of the package.- The inhaler must be used within 3 months of removing it from the aluminum paper wrapper. Write the expiration date (3 months from the opening of the wrapper) in the blank space available on the inhaler label to remind you when to stop using the inhaler.
- The image shows the parts that make up the inhaler, which will already be assembled when you receive it. If the container becomes loose, put it back in the inhaler and continue using it.
Preparing Symbicort
The inhaler needs to be prepared for use in the following situations:
- If you are using your new Symbicort for the first time.
- If you have not used it for more than 7 days.
- If it has been dropped.
To prepare the inhaler for use, follow these instructions:
- Shake the inhaler well for at least 5 seconds to mix the contents of the aerosol cartridge.
- Remove the mouthpiece cap by gently pressing on the protrusions on the side. The cap strap will remain attached to the inhaler.
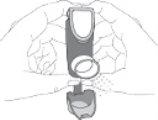
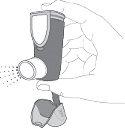 Hold the inhaler in a vertical position. Then, press the counter (on the top of the inhaler) to release a spray into the air. You can use one or both hands, as shown in the photos.
Hold the inhaler in a vertical position. Then, press the counter (on the top of the inhaler) to release a spray into the air. You can use one or both hands, as shown in the photos.- Remove your finger(s) from the counter.
- Wait 10 seconds, shake well, and repeat steps 3 and 4.
- Your inhaler is now ready for use.
How to take an inhalation
Each time you need to take an inhalation, follow these instructions:
- Shake the inhaler well for at least 5 seconds to mix the contents of the aerosol cartridge.
- Remove the mouthpiece cap by gently pressing on the protrusions on the side. Check that the mouthpiece is not blocked.
 Hold the inhaler in a vertical position (you can use one hand or both). Breathe out gently.
Hold the inhaler in a vertical position (you can use one hand or both). Breathe out gently.- Gently place the mouthpiece between your teeth. Close your lips.
- Breathe in slowly and deeply through your mouth. Press the counter (on the top of the inhaler) firmly to release a spray. Hold your breath for a moment while pressing the counter. Breathe in at the same time as you press the counter to ensure the medication reaches your lungs.
- Hold your breath for 10 seconds, or as long as is comfortable for you.
- Before breathing out, release your finger from the counter and remove the inhaler from your mouth. Keep the inhaler in a vertical position.
- Breathe out gently. Before taking another inhalation, shake the inhaler well for at least 5 seconds and repeat steps 3-7.
- Replace the mouthpiece cap.
- Rinse your mouth with water after the morning and evening doses. Do not swallow.
Using a spacer chamber
Your doctor, nurse, or pharmacist may suggest using a spacer chamber (e.g., Aerochamber Plus Flow Vu or Aerochamber Plus). Follow the instructions provided with the spacer chamber.
Cleaning Symbicort
- Clean the inside and outside of the mouthpiece with a dry cloth at least once a week.
- Do not use water or liquids.
- Do not separate the cartridge from the inhaler.
How do I know when to replace my Symbicort inhaler?
- The counter on the top of the inhaler tells you how many inhalations are left in your Symbicort inhaler. It starts with 60 or 120 inhalations when full.
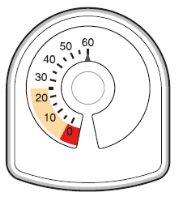
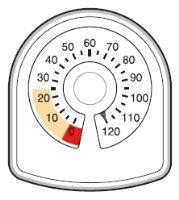
- Each time you take an inhalation, or release a spray into the air, the arrow starts counting down towards zero ('0').
- When the arrow first enters the yellow area, it means there are about 20 inhalations left.
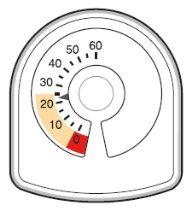
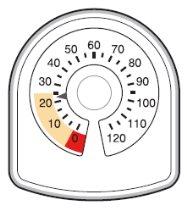
- When the arrow reaches '0', you must stop using your Symbicort inhaler. You may not feel that the inhaler is empty, and it may seem to still be working, but you will not receive the correct amount of medication if you continue using it.
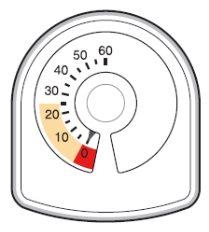
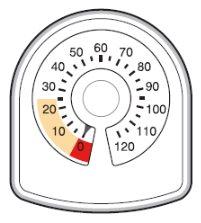
If you use more Symbicort than you should
It is essential to inhale your dose as indicated on the pharmacist's label or as your doctor has recommended. Do not use more doses than prescribed without consulting your doctor.
If you have used more Symbicort than you should, consult your doctor or pharmacist. The most common symptoms and signs that may occur after an overdose are tremors, headache, and rapid heartbeat.
In case of overdose or accidental ingestion, consult the Toxicology Information Service. Phone: 91 5620420, indicating the medication and the amount taken.
If you forget to use Symbicort
- If you forget to take a dose of Symbicort, follow the recommended treatment as soon as you remember. However, if it is almost time for your next dose, do not worry about the missed dose.
- Do nottake a double dose to make up for missed doses.
If you stop using Symbicort
Before stopping the use of Symbicort, you must consult your doctor or pharmacist. If you stop using Symbicort, your asthma symptoms may worsen.
If you have any other questions about using this medication, ask your doctor or pharmacist.
4. Possible side effects
Like all medications, this medication can cause side effects, although not everyone gets them.
If you experience any of the following, stop using Symbicort and consult your doctor immediately:
- Your face, particularly around the mouth (tongue and/or throat), swells, or you experience hives along with difficulty breathing (angioedema) and/or a sudden feeling of fainting, which indicates that you may be having an allergic reaction. This occurs rarely, affecting less than 1 in 1,000 patients.
- You experience wheezing or difficulty breathing immediately after using your Symbicort inhaler. If you experience any of these symptoms, stop using Symbicort immediately and use your separate "reliever inhaler" if available. Consult your doctor immediately, as you may need to change your treatment.This occurs very rarely, affecting less than 1 in 10,000 patients.
Other possible side effects:
Common (may affect up to 1 in 10 patients)
- Palpitations (noticing your heartbeat), tremors, or shivers. These effects, if they occur, are usually mild and disappear as you continue using Symbicort.
- Thrush (fungal infection) in the mouth; this effect is less likely if you rinse your mouth with water after the morning and evening doses of Symbicort.
- Mild throat irritation, cough, hoarseness.
- Headache.
Uncommon (may affect up to 1 in 100 patients)
- Agitation, restlessness, nervousness.
- Difficulty sleeping.
- Dizziness.
- Nausea (discomfort).
- Rapid heartbeat.
- Bruises on the skin.
- Muscle cramps.
- Blurred vision.
Rare (may affect up to 1 in 1,000 patients)
- Rash, itching.
- Bronchospasm (contraction of the airway muscles, causing wheezing). If wheezing occurs suddenly just after using Symbicort, stop using it and consult your doctor immediately.
- Low potassium levels in the blood.
- Irregular heartbeat.
Very rare (may affect up to 1 in 10,000 patients)
- Depression.
- Changes in behavior, especially in children.
- Chest pain or tightness (angina pectoris).
- Increased blood sugar (glucose) levels.
- Changes in taste, such as unpleasant mouth taste.
- Changes in blood pressure.
Inhaled corticosteroids may affect the normal production of steroid hormones in the body, especially if high doses are used for a long time. These effects include:
- changes in bone mineral density (thinning of the bones)
- cataracts (loss of transparency of the lens in the eye)
- glaucoma (increased eye pressure)
- growth delay in children and adolescents
- effects on the adrenal glands (small glands located next to the kidneys).
These effects are much less likely with inhaled corticosteroids than with corticosteroid tablets.
Reporting side effects
If you experience any side effects, consult your doctor or pharmacist, even if they are not listed in this leaflet. You can also report them directly through the Spanish Medicines Monitoring System: www.notificaRAM.es.
By reporting side effects, you can help provide more information on the safety of this medication.
5. Storage of Symbicort
- Keep this medication out of sight and reach of children.
- Do not use this medication after the expiration date stated on the carton, aluminum wrapper, or cartridge label after "EXP". The expiration date is the last day of the month indicated.
- It should be used within 3 months of removing the inhaler from its aluminum wrapper. Write the expiration date (3 months from opening the wrapper) in the blank space available on the inhaler label to remind you when to stop using the inhaler.
- As with most pressurized inhalers, the effect of this medication may decrease when the canister is cold. For best results, this medication should be at room temperature before use.
- Do not refrigerate or freeze. Protect from frost and direct sunlight.
- Always replace the mouthpiece cap and click it into position after using the inhaler.
- Medicines should not be disposed of via wastewater or household waste. Return the inhaler and any unused medication to the pharmacy's SIGRE collection point. Ask your pharmacist how to dispose of the inhaler and any unused medication. This will help protect the environment.
Warning: The cartridge contains a pressurized liquid. Do not expose to temperatures above 50°C. Do not pierce the cartridge. The cartridge should not be broken, punctured, or burned, even when it appears to be empty.
6. Container contents and additional information
Composition of Symbicort
The active ingredients are budesonide and formoterol fumarate dihydrate. Each inhaled dose contains 80 micrograms of budesonide and 2.25 micrograms of formoterol fumarate dihydrate.
The other excipients are apaflurane (HFA 227), povidone, and macrogol. This inhaler does not contain CFCs.
This medication contains fluorinated greenhouse gases. Each inhaler contains 10.8 g of apaflurane (HFC-227ea) which corresponds to 0.035 tons of CO2 equivalent (global warming potential GWP = 3,220).
Appearance of the product and container contents
Symbicort is an inhaler that contains your medication. The medication, a white-colored inhalation suspension, is presented in a pressure-resistant cartridge with an attached dose indicator. The cartridge is mounted on a red plastic adapter with a white plastic mouthpiece and an integrated gray plastic cap. Each inhaler contains 60 or 120 inhalations after it has been prepared for use. Each inhaler is individually packaged in an aluminum foil wrapper containing a desiccant.
Symbicort 80 micrograms/2.25 micrograms/inhalation inhalation suspension in a pressure-resistant container (budesonide/formoterol fumarate dihydrate) is available in containers with a single inhaler.
Only some package sizes may be marketed.
Marketing authorization holder and manufacturer
The holder is:
Laboratory AstraZeneca Pharmaceutical Spain, S.A.
C/ Puerto de Somport 21-23
28050 Madrid
Spain
The manufacturer is:
AstraZeneca Dunkerque Production
224, Avenue de la Dordogne BP 41
59640 Dunkerque (France)
This medication is authorized in the member states of the European Economic Area under the following names:
Austria: Symbicort 80 Mikrogramm/2,25 Mikrogramm/Inhalation Druckgasinhalation, Suspension; Belgium: Symbicort 80 microgram/2,25 microgram/inhalatie, aërosol, suspensie; Symbicort 80 microgrammes/2,25 microgrammes/inhalation, suspension pour inhalation en flacon pressurisé; Symbicort 80 Mikrogramm/2,25 Mikrogramm/Inhalation, Druckgasinhalation, Suspension; Bulgaria: ????????? 80 ??????????/2,25 ??????????/?????????? ????????? ??? ???????? ?? ?????????; Cyprus: Symbicort 80 μικρογραμμ?ρια/2,25 μικρογραμμ?ρια/ψεκασμ?; Croatia: Symbicort 80 mikrograma/2,25 mikrograma po potisku, stlaceni inhalat, suspenzija; Denmark: Symbicort 80 mikrogram/2.25 mikrogram/inhalation; Slovakia: Symbicort 80 mikrogramov/2,25 mikrogramov/inhalacná dávka, inhalacná suspenzia v tlakovom obale; Slovenia: Symbicort 80 mikrogramov/2,25 mikrograma na vdih, inhalacijska suspenzija pod tlakom; Spain: Symbicort 80 microgramos/2,25 microgramos/inhalación suspensión para inhalación en envase a presión; Estonia: Symbicort; Finland: Symbicort 80 mikrog/2.25 mikrog/inhalaatio; France: Symbicort 100/3 microgrammes par inhalation, suspension pour inhalation en flacon préssurisé; Greece: Symbicort 80 μικρογραμμ?ρια/2,25 μικρογραμμ?ρια/ψεκασμ?; Netherlands: Symbicort aërosol 100/3, 100 microgram/3 microgram per dosis, aërosol, suspensie; Hungary: Symbicort 2,25 mikrogramm/80 mikrogramm/ adag túlnyomásos inhalációs szuszpenzió; Iceland: Symbicort 80 míkrógrömm/2,25 míkrógrömm/ inhalation; Italy: Symbicort; Latvia: Symbicort 80 mikrogrami/2.25 mikrogrami/ inhalacija, izsmidzinajuma, aerosols inhalacijam zem spiediena; Lithuania: Symbicort 80 mikrogramu/2,25 mikrogramo/išpurškime suslegtoji ikvepiamoji suspensija; Luxembourg: Symbicort 80 microgrammes/2,25 microgrammes/inhalation, suspension pour inhalation en flacon pressurisé; Malta: Symbicort 100 micrograms/3 micrograms/inhalation, pressurised inhalation, suspension; Norway: Symbicort 80 mikrogram/2.25 mikrogram/ inhalasjon; Poland: Symbicort; Portugal: Symbicort 80 microgramas/2,25 microgramas/inalação Suspensão pressurizada para inalação; United Kingdom: Symbicort 100 micrograms/3 micrograms per actuation pressurised inhalation, suspension; Czech Republic: Symbicort 80 micrograms/2,25 micrograms/inhalation, suspension in pMDI; Romania: Symbicort 80 micrograme/2,25 micrograme/inhala?ie, suspensie de inhalat presurizata; Sweden: Symbicort 80 mikrogram/2.25 mikrogram/inhalation
Date of the last revision of this prospectus: January 2025
Detailed and updated information on this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Average pharmacy price19.78 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to SYMBICORT 80 micrograms/2.25 micrograms/inhalation pressurized suspension for inhalationDosage form: PULMONARY INHALATION, 160 micrograms / 4.5 microgramsActive substance: formoterol and budesonideManufacturer: Teva Pharma B.V.Prescription requiredDosage form: PULMONARY INHALATION, 320 micrograms / 9 microgramsActive substance: formoterol and budesonideManufacturer: Teva Pharma B.V.Prescription requiredDosage form: PULMONARY INHALATION, 160 MICROGRAMS/4.5 MICROGRAMSActive substance: formoterol and budesonideManufacturer: Cipla Europe N.V.Prescription required
Online doctors for SYMBICORT 80 micrograms/2.25 micrograms/inhalation pressurized suspension for inhalation
Discuss questions about SYMBICORT 80 micrograms/2.25 micrograms/inhalation pressurized suspension for inhalation, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















