
SUPREFACT DEPOT 9.45 mg IMPLANT

How to use SUPREFACT DEPOT 9.45 mg IMPLANT
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Suprefact Depot 9.45 mg implant
Buserelin
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again. If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Suprefact Depot and what is it used for
- What you need to know before you use Suprefact Depot
- How to use Suprefact Depot
- Possible side effects
- Storage of Suprefact Depot
- Contents of the pack and other information
1. What is Suprefact Depot and what is it used for
What is Suprefact Depot
Suprefact Depot contains the active substance buserelin. Buserelin is similar to a hormone that is produced naturally in the brain. It belongs to a group of medicines called gonadotropin-releasing hormone (GnRH) analogues.
How Suprefact Depot works
It works by reducing the amount of hormones that stimulate the growth of prostate tumors. The prostate is a gland that is located below the bladder in men.
What Suprefact Depot is used for
Suprefact Depot is used for the treatment of advanced prostate cancer.
2. What you need to know before you use Suprefact Depot
Do not use Suprefact Depot:
- if you are allergic to buserelin, to other GnRH analogues (such as leuprolide, goserelin, triptorelin) or to any of the other ingredients of this medicine (listed in section 6).
The symptoms of an allergic reaction could be: skin rash, swallowing or breathing problems, swelling of the lips, face, throat or tongue.
Do not take this medicine if you have any of the above. If you are not sure, ask your doctor or pharmacist before starting treatment with Suprefact Depot.
Warnings and precautions
Tell your doctor or pharmacist before you start using Suprefact Depot if:
- you have had your testicles removed
- you have cancer that has spread (metastatic cancer). At the beginning, it is important for you to use another medicine to reduce the levels of certain hormones. However, this may cause tumor pain; if this happens, ask your doctor or pharmacist
- you have difficulty urinating
- you have risk factors for cardiovascular disease or diabetes
- you have a heart condition or are being treated for it, including medicines to control your heart rhythm (arrhythmias). The risk of heart rhythm problems may increase when using Suprefact Depot
- you have diabetes. Check your blood sugar levels regularly. This is because Suprefact Depot affects metabolism and therefore your blood sugar levels
- you have high blood pressure. Your doctor or nurse should check your blood pressure regularly. This is because Suprefact Depot affects blood pressure
- you have had depression in the past. You should be closely monitored for your mental state because there is a risk that depression could occur again or worsen
- you have a reduced number of red blood cells or feel an increase in tiredness (anemia).
If you are not sure if any of the above applies to you, ask your doctor or pharmacist before using Suprefact Depot.
Your doctor will monitor your bone density and may prescribe you the appropriate treatment. This is because the use of GnRH analogues can cause a decrease in bone density, osteoporosis (weakening of the bones) and an increased risk of bone fractures, especially if you have risk factors such as chronic alcoholism, smoking, or if there is a history of osteoporosis in your family, or if you have been taking anticonvulsant or corticosteroid medicines for a long time.
There have been reports of depression in patients using Suprefact Depot, which could be severe. If you are using Suprefact Depot and develop a depressive mood, inform your doctor.
Other medicines and Suprefact Depot
Tell your doctor or pharmacist if you are using, have recently used or might use any other medicines. This includes medicines bought without a prescription and herbal medicines. This is because Suprefact Depot may affect the way these medicines work. Other medicines may also affect the way Suprefact Depot works.
In particular, tell your doctor:
- if you are taking medicines for diabetes. This is because Suprefact Depot may affect the way these medicines work, which may lead to a worsening of diabetes.
- if you are using medicines used to treat heart rhythm problems (such as quinidine, procainamide, amiodarone and sotalol). This is because Suprefact Depot may interfere with these medicines.
- if you are using other medicines (such as methadone (used for pain relief and for detoxification from other medicines), moxifloxacin (an antibiotic), antipsychotics (used for severe mental illnesses). This is because Suprefact Depot may increase the risk of heart rhythm problems when used with these medicines.
Pregnancy, breast-feeding and fertility
Suprefact Depot is a medicine that should only be used by men. It should not be used by women.
Driving and using machines
After taking this medicine, you may experience some side effects. Some of these side effects (such as dizziness) may affect your ability to concentrate and react. If this happens, be careful when driving, using tools or machines, or when performing any task that requires a high level of attention.
3. How to use Suprefact Depot
The contents of a pre-filled syringe (which contains 3 cylindrical implants with a final dose of 9.45 mg of buserelin) are injected under the skin (subcutaneously) in the stomach area, every 3 months. This time can be extended by up to 3 weeks.
The injection site should be disinfected. The injection is usually given by a doctor or nurse. Before use, the implant should be at room temperature. A local anesthetic may be applied to alleviate the pain of the implant injection. Follow your doctor's instructions about when to use Suprefact Depot and the time that should pass between each injection.
Blood tests
- Your doctor will perform blood tests to check if this medicine is working properly.
If you use more Suprefact Depot than you should
It is unlikely that your doctor or nurse will give you more medicine than you should. If more medicine than necessary is used, you may feel weakness, nervousness, dizziness or nausea. You may also feel headache, hot flashes, stomach pain, swelling (edema) in the ankles and legs, breast enlargement, or reactions at the injection site.
Your doctor will give you the appropriate treatment for these side effects.
In case of overdose, consult your doctor or pharmacist or call the Toxicology Information Service, phone: 91 562 04 20, indicating the medicine and the amount used.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
If you experience any side effects, it is important that you inform your doctor before the next treatment with Suprefact Depot.
If you experience a severe allergic reaction such as shortness of breath or shock, please contact your doctor immediately as it may be necessary to remove the implant.
Side effects that may occur at the start of your treatment
At the beginning of your treatment, the amount of sex hormones that your body produces may increase and you may notice a temporary worsening of symptoms. For example, you may experience bone pain, muscle weakness in the legs, difficulty urinating, fluid retention or blood clotting problems in the lungs. To prevent this, other medicines such as cyproterone acetate are usually given. Treatment with cyproterone should continue for 3-4 weeks after receiving Suprefact Depot. After this time, testosterone levels usually decrease to the desired values in response to Suprefact Depot.
Tell your doctor or pharmacist if any of the following side effects are severe or last more than a few days:
Common (may affect up to 1 in 10 people)
- Loss of sexual desire (libido)
- Difficulty getting an erection
- Headache
- Hot flashes
- Decrease in testicle size (testicular atrophy)
- Pain or other reactions at the injection site (such as redness or swelling)
- Mood changes, depression (long-term treatment).
Uncommon (may affect up to 1 in 100 people)
- Allergic reactions such as skin rashes that may be red and itchy (including hives)
- Drowsiness and fatigue
- Dizziness
- Constipation
- Breast enlargement
- Fluid retention (edema) around the ankles and legs
- Increased liver enzymes that show up in the results of some blood tests
- Weight changes
- Mood changes, depression (short-term treatment).
Rare (may affect up to 1 in 1,000 people)
- Severe allergic reactions such as shortness of breath
- Nervousness, stress, and emotional instability. Additionally, difficulty sleeping and memory or concentration problems
- Fast or irregular heartbeats (palpitations), increased blood pressure in people who already have high blood pressure (hypertension)
- Dizziness (nausea and vomiting) or diarrhea
- Increased or decreased hair and body hair
- Changes in blood lipids and increased bilirubin that show up in the results of some blood tests.
Very rare (may affect up to 1 in 10,000 people)
- Severe allergic reactions with shock
- Increased thirst, changes in appetite, decreased glucose tolerance (in diabetic patients, this may lead to a loss of diabetic control)
- Ringing in the ears (tinnitus) and hearing disorders
- Vision changes such as blurred vision and feeling of pressure in the eye
- Muscle or bone pain
- General deterioration
- Decrease in the number of blood cells that may cause abnormalities in blood test results and/or bruising (bruises)
- Increased size of benign tumors in the pituitary gland or temporary increase in tumor pain.
Frequency not known (cannot be estimated from the available data)
- Changes in the electrocardiogram (ECG) (prolongation of the QT interval).
With other presentations that contain buserelin, abnormal skin sensations such as tingling have also been observed.
This group of medicines (GnRH analogues) may cause a decrease in bone density, osteoporosis, and an increased risk of bone fractures. The risk of bone fractures increases over time. GnRH analogues may increase the risk of cardiovascular disease (such as heart attack and stroke), diabetes, or anemia (decrease in the number of red blood cells that makes you feel tired).
Reporting of side effects:
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly to the Spanish Medicines and Healthcare Products Agency (AEMPS) through the website: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Suprefact Depot
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton after EXP. The expiry date refers to the last day of that month.
Store in a refrigerator (between 2°C and 8°C). It can be stored at a maximum temperature of 25°C for a maximum of 7 days.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
Composition of Suprefact Depot
The active substance of Suprefact Depot is buserelin. Each pre-filled syringe is loaded with 1 implant consisting of 3 cylindrical rods, with a final dose of 9.9 mg of buserelin acetate. This is equivalent to 9.45 mg of buserelin.
The other ingredient is Poly (D, L-lactic-co-glycolic acid) with a ratio of 75:25 of lactic to glycolic acid.
Appearance and packaging
A pack contains 1 or 2 pre-filled syringes.
Each syringe contains 3 cream-colored cylindrical implants.
Marketing authorisation holder and manufacturer
Marketing authorisation holder
CHEPLAPHARM Arzneimittel GmbH
Ziegelhof 24
17489 Greifswald
Germany
Manufacturer
Sanofi Aventis Deutschland GmbH
Brüningstraße 50 – Frankfurt am Main
65926 Germany
Local representative
Laboratorios Rubió, S.A.
Industria, 29
Pol. Ind. Comte de Sert
08755 Castellbisbal (Barcelona)
Spain
This medicine is authorised in the Member States of the European Economic Area under the following names:
Austria: Suprefact Depot - Implantat Für 3 Monate
Belgium: Suprefact Depot 9.45 mg Implant
Denmark: Suprefact Depot
Finland: Suprefact Depot 9.45 mg implantaatti
France: Trigonist 9.45 mg implant pour voie sous-cutanée
Germany: Profact Depot 9,45 mg 3-Monatsimplantat
Italy: Suprefact depot 3 Mesi
Luxembourg: Suprefact Depot 9.45 mg Implant
Netherlands: Suprefact Depot 3 Maanden, implantatiestift 9.45 mg
Portugal: Suprefact Depot 3 Meses
Sweden: Suprefact Depot 9.45 mg implantat
United Kingdom: Suprefact Depot 9.45 mg implant, for subcutaneous route
Date of last revision of this leaflet:June 2015
Other sources of information
Detailed information on this medicine is available on the website of the Spanish Agency for Medicines and Healthcare Products (AEMPS) http://www.aemps.gob.es/.
This information is intended only for healthcare professionals.
1Medicine name
Suprefact Depot 9.45 mg implant, subcutaneous route
2DOSAGE AND ADMINISTRATION
A preloaded syringe with 1 implant contains 3 cylinders, which are injected under the skin of the abdomen every three months. It is essential to maintain the administration rhythm every three months regularly; however, the injection interval could occasionally be extended up to 3 weeks. Before injection, a local anesthetic may be administered.
Before use, the implant must be at room temperature.
Warning. To avoid the 3 cylinders of the implant falling from the injection needle (A), keep the applicator in a vertical position until immediately before the puncture, with the needle pointing upwards.
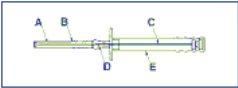
A: Needle
B: Protective cap of the needle
C: Plunger
D: Implant
E: Protective sheath of the plunger
- After opening the package and removing the applicator from its container, check that the 3 cylinders of the implant are situated in the applicator window. If necessary, gently tap the protective cap of the syringe with your finger to reposition them in the window. Once the package is opened, the applicator must be used immediately.
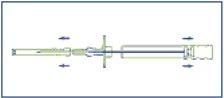
- Disinfect the injection area located on one side of the abdominal wall. First, remove the protective sheath of the plunger (E), and then remove the protective cap of the injection needle (B).

- Pinch a skin fold and insert the needle approximately 3 cm (just over an inch) into the subcutaneous tissue. Keep the applicator in a horizontal position at the moment before the puncture or with the tip of the needle slightly oriented upwards. Remove the applicator 1-2 cm approximately before the injection of the cylinders.
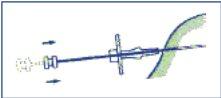
- Inject the three cylinders of the implant into the subcutaneous tissue by pushing the plunger completely. Compress the puncture canal while withdrawing the needle so that the 3 cylinders of the implant remain retained in the tissue.

- To ensure that the three cylinders of the implant have been injected, check that the end of the plunger is visible at the end of the needle.
It is recommended to administer an antiandrogen 5 days before starting treatment with Suprefact Depot.
3PHARMACEUTICAL DATA
3.1List of excipients
Poly (D, L-lactide-co-glycolide)
3.2Incompatibilities
Not applicable since the product is presented in a special applicator.
3.3Shelf life
3 years.
3.4Special precautions for storage
Store in the refrigerator (between 2°C and 8°C). It can be stored at a maximum temperature of 25°C for a maximum of 7 days.
3.5Nature and content of the container
Preloaded syringe with a cylindrical implant composed of three cylinders, housed in a disposable applicator made of cellulose propionate and stainless steel, sealed in a laminated bag made of polyethylene terephthalate, aluminum, and low-density polyethylene.
Presentation: 1 or 2 preloaded syringes per package.
Only some package sizes may be marketed.
Detailed and updated information on this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Average pharmacy price341.13 EUR
- Availability in pharmacies
Supply issue reported
Data from the Spanish Agency of Medicines (AEMPS) indicates a supply issue affecting this medicine.<br><br>Availability may be limited in some pharmacies.<br><br>For updates or alternatives, consult your pharmacist. - Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to SUPREFACT DEPOT 9.45 mg IMPLANTDosage form: INJECTABLE, 1 mg/mlActive substance: buserelinManufacturer: Cheplapharm Arzneimittel GmbhPrescription requiredDosage form: INJECTABLE, 42 mgActive substance: leuprorelinManufacturer: Accord Healthcare S.L.U.Prescription requiredDosage form: INJECTABLE, 0.1 mgActive substance: triptorelinManufacturer: Ipsen Pharma S.A.Prescription required
Online doctors for SUPREFACT DEPOT 9.45 mg IMPLANT
Discuss questions about SUPREFACT DEPOT 9.45 mg IMPLANT, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions












