
ZOLMITRIPTAN FLAS SANDOZ 5 mg ORALLY DISINTEGRATING TABLETS

How to use ZOLMITRIPTAN FLAS SANDOZ 5 mg ORALLY DISINTEGRATING TABLETS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Zolmitriptan Flas Sandoz 2.5 mg Orodispersible Tablets EFG
Zolmitriptan Flas Sandoz 5 mg Orodispersible Tablets EFG
Read the entire package leaflet carefully before you start taking this medicine, as it contains important information for you.
- Keep this package leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others, as it may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this package leaflet. See section 4.
Contents of the Package Leaflet:
- What is Zolmitriptan Flas Sandoz and what is it used for
- What you need to know before you take Zolmitriptan Flas Sandoz
- How to take Zolmitriptan Flas Sandoz
- Possible side effects
- Storage of Zolmitriptan Flas Sandoz
- Contents of the pack and other information
1. What is Zolmitriptan Flas Sandoz and what is it used for
Zolmitriptan Flas Sandoz contains the active substance zolmitriptan and belongs to a group of medicines called triptans.
Zolmitriptanis used to treat migraine headaches in adults over 18 years of age.
- The symptoms of migraine can be caused by the dilation of blood vessels in the head. It is thought that zolmitriptan reduces the dilation of these blood vessels. This helps to relieve the headache and other symptoms of a migraine attack, such as feeling sick (nausea or vomiting) and sensitivity to light and sound.
- Zolmitriptan only works when a migraine attack has already started. It will not prevent you from getting a migraine attack.
2. What you need to know before you take Zolmitriptan Flas Sandoz
Do not take Zolmitriptan Flas Sandoz if:
- you are allergic to zolmitriptan or any of the other ingredients of this medicine (listed in section 6),
- you have severe kidney problems,
- you have had a stroke (cerebrovascular accident or CVA) or symptoms of short duration similar to those of a stroke (transient ischaemic attack or TIA),
- you have moderate or severe high blood pressure, or mild high blood pressure that is not controlled with medication,
- you have had heart problems, including heart attack, angina (chest pain caused by exercise or effort), or a special type of chest pain known as Prinzmetal's angina, or have experienced symptoms related to the heart such as shortness of breath or pressure on the chest,
- you have had problems with blood flow to your legs (peripheral vascular disease),
- you are taking another medicine for migraine, such as ergotamine, ergot-type medicines (dihydroergotamine, methysergide), or another medicine from the same group as zolmitriptan (i.e. 5-HT1B/1D receptor agonist or triptan, such as sumatriptan, naratriptan, or rizatriptan) (see section "Other medicines and Zolmitriptan Flas Sandoz").
Warnings and Precautions
Talk to your doctor or pharmacist before taking zolmitriptan if you have:
- any risk factors for heart disease (poor blood flow to the heart):
- high blood pressure or diabetes,
- high levels of cholesterol in the blood,
- you smoke,
- you have a family history of heart disease,
- you are a man over 40 years old or a post-menopausal woman,
- a type of abnormal heartbeat (Wolff-Parkinson-White syndrome) or any other type of heart rhythm disorder,
- liver or kidney problems,
- headache accompanied by dizziness, difficulty walking, lack of coordination, or weakness in the arms or legs.
Tell your doctor if you are taking any medicines for depression or herbal remedies such as St. John's Wort (Hypericum perforatum) (for more information, see section "Other medicines and Zolmitriptan Flas Sandoz").
Zolmitriptan may increase blood pressure. If blood pressure rises too high, you may experience symptoms such as headache, dizziness, or ringing in the ears. If this happens, contact your doctor.
If you take zolmitriptan too frequently, you may get chronic headaches. In this case, you should contact your doctor, as you may need to stop taking this medicine.
Tell your doctor about your symptoms. Your doctor will tell you if you have migraine. You should only take zolmitriptan for a migraine attack. Do not use zolmitriptan to treat headaches that may be caused by other, more serious diseases.
Zolmitriptan Flas Sandoz is not recommended for people over 65 years old. If you are over 65, your doctor will tell you if you can take these tablets.
Children and Adolescents
Zolmitriptan Flas Sandoz is not recommended for people under 18 years old.
Other Medicines and Zolmitriptan Flas Sandoz
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines.
Do not take Zolmitriptan Flas Sandoz with other medicines for migraine, such as:
- other medicines from the same group as zolmitriptan (e.g. 5-HT1B/1D receptor agonists or triptans, e.g. sumatriptan, naratriptan, or rizatriptan).
If you take other triptans different from zolmitriptan, wait 24 hours before taking Zolmitriptan Flas Sandoz.
After taking zolmitriptan, wait 24 hours before taking other triptans different from Zolmitriptan Flas Sandoz,
- ergotamine-derived medicines, dihydroergotamine, and methysergide. After taking zolmitriptan, you should wait at least 6 hours before taking these types of medicines, and if you are taking ergotamine-derived medicines, you should wait at least 24 hours before taking zolmitriptan.
Consult your doctor about the instructions for taking the medicine and the risks of taking these tablets with:
- medicines for depression:
- monoamine oxidase inhibitors (MAOIs), such as moclobemide,
- selective serotonin reuptake inhibitors (SSRIs), such as sertraline, escitalopram, fluoxetine, and fluvoxamine,
- serotonin and norepinephrine reuptake inhibitors (SNRIs), such as venlafaxine and duloxetine,
Serotonin syndrome is a rare but potentially life-threatening disorder that has been reported in some patients who took zolmitriptan in combination with serotonergic medicines (e.g. certain medicines for depression). The symptoms of serotonin syndrome can be, for example, agitation, tremors, restlessness, fever, excessive sweating, spasms, muscle rigidity, uncoordinated movements of the limbs or eyes, and involuntary muscle contractions. Your doctor can give you more information.
- cimetidine (for indigestion or stomach ulcers),
- quinolone-type antibiotics (such as ciprofloxacin),
- herbal remedies such as St. John's Wort (Hypericum perforatum), as taking it with zolmitriptan increases the likelihood of side effects. It is not recommended to take zolmitriptan and St. John's Wort at the same time.
Pregnancy and Breastfeeding
If you are pregnant, or think you may be pregnant, or are planning to have a baby, ask your doctor for advice before taking this medicine. If you are pregnant, you should only take zolmitriptan if your doctor decides that it is necessary.
If you are breastfeeding, ask your doctor for advice before taking this medicine. You should avoid breastfeeding for 24 hours after taking zolmitriptan.
Driving and Using Machines
Migraine itself or treatment with zolmitriptan may cause drowsiness in some patients. There have also been reports of dizziness in some patients treated with this medicine. If you experience these effects, you should be careful when driving or using machines.
Zolmitriptan Flas Sandoz Contains Sodium, Sulphites, and Aspartame
This medicine contains less than 23 mg of sodium (1 mmol) per orodispersible tablet; this is essentially "sodium-free".
Sulphites can rarely cause severe allergic reactions and bronchospasm (sudden feeling of suffocation).
Zolmitriptan Flas Sandoz 2.5 mg Orodispersible Tablets EFG
This medicine contains 2.5 mg of aspartame in each orodispersible tablet. Aspartame is a source of phenylalanine that may be harmful if you have phenylketonuria (PKU), a rare genetic disorder in which phenylalanine accumulates because the body cannot eliminate it properly.
Zolmitriptan Flas Sandoz 5 mg Orodispersible Tablets EFG
This medicine contains 5 mg of aspartame in each orodispersible tablet. Aspartame is a source of phenylalanine that may be harmful if you have phenylketonuria (PKU), a rare genetic disorder in which phenylalanine accumulates because the body cannot eliminate it properly.
3. How to Take Zolmitriptan Flas Sandoz
Always take this medicine exactly as your doctor or pharmacist has told you. If you are not sure, check with your doctor or pharmacist.
You can take Zolmitriptan Flas Sandoz as soon as a migraine headache starts. You can also take it once the headache has started.
The recommended doseis one 2.5 mg tablet.
Your doctor will tell you which dose is best for you and it is important that you take the medicine as your doctor has told you.
Most migraine attacks are relieved by a single dose (one tablet) of zolmitriptan, but if this is not the case, do not take a second tablet to treat the same attackas it is unlikely to work.
Talk to your doctor if the tablets do not give sufficient relief from your migraine. Your doctor may increase the dose to 5 mg or change the treatment.
If you get anothermigraine attack within the first 24 hours after the first one, you can take another tablet of zolmitriptan, but you should nevertake more than two tablets in 24 hours.
If you have been prescribed the 2.5 mg tablet, the maximum daily dose is 5 mg.
If you have been prescribed the 5 mg tablet, the maximum daily dose is 10 mg.
In either case, you should wait at least 2 hours between doses.
Method of Administration
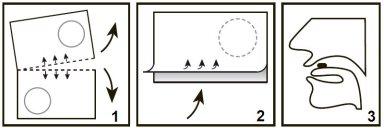
Take the orodispersible tablets as follows:
- Separate the blister along the perforation.
- Pull the blister strip carefully from the arrow, as shown in the diagram.
- Place the tablet on the tongue, where it will dissolve and can be swallowed with the saliva. You do not need to drink water to swallow the tablet.
You can take Zolmitriptan Flas Sandoz with or without food. This does not affect how zolmitriptan works.
If you take more Zolmitriptan Flas Sandoz than you should
In case of overdose or accidental ingestion, talk to your doctor or pharmacist immediately or call the Toxicology Information Service, telephone: 91 562 04 20, indicating the medicine and the amount taken.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible Side Effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
If you experience any of the following side effects, stop taking the medicine and talk to your doctor immediately.
Severe side effects (rare: may affect up to 1 in 1,000 people):
- allergic reactions, sometimes very severe, such as swelling of the face, lips, mouth, tongue, and throat, which can cause difficulty breathing, speaking, or swallowing.
Very severe side effects (very rare: may affect up to 1 in 10,000 people):
- chest pain or tightness, or throat, shortness of breath, or other symptoms compatible with a heart attack,
- spasm of the blood vessels of the digestive tract, which can cause damage to your digestive tract. You may feel stomach pain or have bloody diarrhea,
Other possible side effects
Common:may affect up to 1 in 10 people.
- headache,
- tingling sensation, skin hypersensitivity,
- numbness, dizziness, or feeling of warmth,
- irregular or rapid heartbeats,
- nausea (general discomfort), vomiting, stomach pain,
- dry mouth,
- weakness or muscle pain,
- feeling of weakness,
- heaviness, pressure, or pain in the throat, neck, arms, and legs, or chest,
- difficulty swallowing.
Uncommon:may affect up to 1 in 100 people.
- rapid heartbeats,
- mildly increased blood pressure or short-term high blood pressure,
- increased amount of urine or number of times you need to urinate.
Rare:may affect up to 1 in 1,000 people.
- itchy rash (urticaria).
Very rare:may affect up to 1 in 10,000 people.
- sudden and urgent need to urinate.
As with other medicines of this type, there have been very rare reports of heart attacks and strokes, most of which occurred in patients with risk factors for heart and blood vessel disease (high blood pressure, diabetes, smoking, family history of heart disease or stroke).
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this package leaflet.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this package leaflet. You can also report side effects directly via the Spanish Medicines Monitoring System for Human Use: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Zolmitriptan Flas Sandoz
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the packaging and blister, after EXP. The expiry date is the last day of the month shown.
Store in the original packaging to protect from moisture.
Medicines should not be disposed of via wastewater or household waste. Return any unused medicine to a pharmacy for disposal. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Packaging Contents and Additional Information
Zolmitriptan Flas Sandoz Composition
- The active ingredient is zolmitriptan.
Zolmitriptan Flas Sandoz 2.5 mg orodispersible tablets EFG
Each orodispersible tablet contains 2.5 mg of zolmitriptan.
Zolmitriptan Flas Sandoz 5 mg orodispersible tablets EFG
Each orodispersible tablet contains 5 mg of zolmitriptan.
- Other components: are silicified microcrystalline cellulose, crospovidone, sodium hydrogen carbonate, anhydrous citric acid, anhydrous colloidal silica, mannitol (E421), sweet orange flavor (contains sodium, sulfites, and propylene glycol), aspartame (E951) (see additional information at the end of section 2), and magnesium stearate.
Product Appearance and Packaging Contents
Zolmitriptan Flas Sandoz 2.5 mg orodispersible tablets EFG
Orodispersible tablets, white, round, flat, and engraved with "ZMT 2.5" on one side.
Zolmitriptan Flas Sandoz 5 mg orodispersible tablets EFG
Orodispersible tablets, white, round, flat, and engraved with "ZMT 5" on one side.
The orodispersible tablets are packaged in Alu/Alu blisters, inserted into a cardboard box.
Zolmitriptan 2.5 mg orodispersible tablets
Package sizes: 2, 3, 4, 6, 10, 12, 18, or 24 orodispersible tablets.
Zolmitriptan 5 mg orodispersible tablets
Package sizes: 2, 3, 4, 6, 12, 18, or 24 orodispersible tablets.
Only some package sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder:
Sandoz Farmacéutica, S.A.
Centro Empresarial Parque Norte
Edificio Roble
C/ Serrano Galvache, 56
28033 Madrid
Spain
Manufacturer:
Salutas Pharma GmbH
Otto-von-Guericke-Allee 1,
39179 Barleben
Germany
Salutas Pharma GmbH
Dieselstrasse 5, 70839 Gerlingen
Germany
or
Lek Pharmaceuticals d.d.
Verovškova 57,
1526 Ljubljana
Slovenia
or
Lek Pharmaceuticals d.d.
Trimlini 2D, 9220
Lendava
Slovenia
or
Lek S.A.
Ul. Domaniewska 50 C,
02-672 Warsaw
Poland
or
S.C. Sandoz, S.R.L.
Str. Livezeni nr. 7A,
RO-540472 Targu-Mures
Romania
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Belgium | Zolmitriptan Sandoz 2.5 mg, orodispersible tablets |
Finland | Zolmitriptan Sandoz 2.5 mg suussa hajoava tabletti |
France | ZOLMITRIPTAN SANDOZ 2.5 mg, comprimé orodispersible |
Norway | Zolmitriptan Sandoz 2.5 mg smeltetablett |
Netherlands | Zolmitriptan Sandoz smelttablet 2.5 mg, orodispergeerbare tabletten Zolmitriptan Sandoz smelttablet 5 mg, orodispergeerbare tabletten |
Date of the last revision of this leaflet:October 2021.
Detailed and updated information on this medicinal product is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Average pharmacy price32.16 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ZOLMITRIPTAN FLAS SANDOZ 5 mg ORALLY DISINTEGRATING TABLETSDosage form: ORALLY DISINTEGRATING TABLET/LIOTAB, 2.5 mg/ tabletActive substance: zolmitriptanManufacturer: Laboratorios Cinfa S.A.Prescription requiredDosage form: ORALLY DISINTEGRATING TABLET/LYOTAB, 5 mg/ tabletActive substance: zolmitriptanManufacturer: Laboratorios Cinfa S.A.Prescription requiredDosage form: ORALLY DISINTEGRATING TABLET/LIOTAB, 2.5 mgActive substance: zolmitriptanManufacturer: Laboratorios Combix S.L.U.Prescription required
Online doctors for ZOLMITRIPTAN FLAS SANDOZ 5 mg ORALLY DISINTEGRATING TABLETS
Discuss questions about ZOLMITRIPTAN FLAS SANDOZ 5 mg ORALLY DISINTEGRATING TABLETS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















