
Zolmiles

Ask a doctor about a prescription for Zolmiles

How to use Zolmiles
1. What is Zolmiles and what is it used for
Zolmiles contains zolmitriptan and belongs to a group of medicines called triptans.
Zolmiles is used to treat migraine headaches.
The symptoms of migraine can be caused by the expansion of blood vessels in the head. It is thought that Zolmiles narrows the expanded blood vessels. This in turn helps to reduce the headache and other symptoms of a migraine attack, such as nausea or vomiting and sensitivity to light and sound.
Zolmiles only works at the start of a migraine attack. It will not prevent an attack from occurring.
2. Important information before taking Zolmiles
When not to take Zolmiles:
If the patient is unsure whether any of the above applies to them, they should consult their doctor or pharmacist.
Warnings and precautions
Before starting to take Zolmiles, the patient should discuss it with their doctor or pharmacist if:
- the patient is at risk of ischaemic heart disease (reduced blood flow through the heart's blood vessels). The risk is greater if the patient smokes, has high blood pressure, high cholesterol, diabetes or if there have been cases of ischaemic heart disease in the patient's family
- the patient has been informed that they have Wolff-Parkinson-White syndrome (a type of heart rhythm disorder)
- the patient has ever had liver problems
- the patient has headaches that are not typical migraines
- the patient is taking other medicines for depression (see the section "Zolmiles and other medicines" below).
If Zolmiles is taken at the same time as medicines from the SSRI (selective serotonin reuptake inhibitors) or SNRI (serotonin-norepinephrine reuptake inhibitors) groups used to treat depression, there is a risk of serotonin syndrome. Symptoms can be severe and include shivering, overactivity, nausea, fever, excessive sweating, hallucinations, confusion and coma. If the patient is taking combined therapy, the doctor should carefully monitor the patient's condition, especially at the start of treatment, when the dose is increased or when other serotoninergic medicines are added to the treatment. If the patient experiences any of these symptoms, they should contact their doctor as soon as possible.
As with other anti-migraine treatments, taking too much zolmitriptan may cause daily headaches or worsen migraine headaches. If the patient suspects this is the case, they should consult their doctor. To resolve the problem, it may be necessary to stop taking zolmitriptan.
In the event of hospitalization, the patient should inform the hospital staff that they are taking Zolmiles.
Children and adolescents
Zolmiles is not recommended for patients under 18 years of age.
Elderly
Zolmiles is not recommended for patients over 65 years of age.
Zolmiles and other medicines
The patient should tell their doctor or pharmacist about all medicines they are taking, have recently taken or might take.
In particular, the patient should tell their doctor if they are taking any of the following medicines:
Medicines used to treat migraine
Medicines used to treat depression (see also the section above "Warnings and precautions")
- moclobemide or fluvoxamine
- medicines called selective serotonin reuptake inhibitors (SSRIs)
- medicines called selective norepinephrine reuptake inhibitors (SNRIs), such as venlafaxine, duloxetine.
Other medicines
- cimetidine (used for indigestion or stomach ulcers)
- quinolone antibiotics (such as ciprofloxacin).
If the patient is taking herbal preparations containing St. John's Wort (Hypericum perforatum), the risk of adverse reactions to Zolmiles is higher.
Zolmiles with food and drink
Zolmiles can be taken with or without food. Meals do not affect the action of Zolmiles.
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks they may be pregnant or are planning to have a baby, they should consult their doctor or pharmacist before taking this medicine.
It is not known whether taking Zolmiles during pregnancy harms the baby.
The patient should not breastfeed for 24 hours after taking Zolmiles.
Driving and using machines
During a migraine attack, the patient's reactions may be slower than usual. This should be taken into account when driving, using tools or operating machinery.
It is unlikely that Zolmiles will affect the ability to drive, use tools or operate machinery. However, it is best to wait and see how Zolmiles affects the patient before performing these activities.
Zolmiles contains glucose (maltodextrin component)
If the patient has previously been diagnosed with intolerance to some sugars, they should consult their doctor before taking Zolmiles.
Zolmiles contains aspartame (E951)
Each 2.5 mg oral dispersible tablet of Zolmiles contains 4 mg of aspartame.
Each 5 mg oral dispersible tablet of Zolmiles contains 8 mg of aspartame.
Aspartame is a source of phenylalanine. It may be harmful to patients with phenylketonuria (PKU).
This is a rare genetic disorder in which phenylalanine accumulates in the body due to its impaired elimination.
Zolmiles contains sodium
The medicine contains less than 1 mmol (23 mg) of sodium per oral dispersible tablet, which means that the medicine is considered "sodium-free".
3. How to take Zolmiles
This medicine should always be taken exactly as prescribed by the doctor or pharmacist. If the patient is unsure, they should ask their doctor or pharmacist.
Zolmiles should be taken after the first symptoms of a migraine attack appear. It can also be taken during an attack.
The recommended dose is one tablet (2.5 mg or 5 mg).
A second tablet can be taken if the migraine does not improve after 2 hours orif it returns within 24 hours.
If the tablets do not bring significant improvement in migraine symptoms, the patient should consult their doctor. The doctor may recommend increasing the dose to 5 mg or changing the treatment.
The patient should not take a higher dose than recommended.
The patient should not take more than two doses in 24 hours. If 2.5 mg tablets have been prescribed, the maximum daily dose is 5 mg. If 5 mg tablets have been prescribed, the maximum daily dose is 10 mg.
Instructions for use
- 1. Do not push the tablet through the foil (Figure 1).
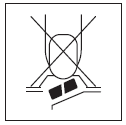
Figure 1
- 2. Tear off one blister pack (Figure 2).
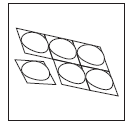
Figure 2
- 3. Carefully peel off the foil covering, starting from the place indicated by the arrow (Figures 3 and 4).
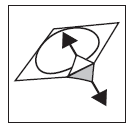
Figure 3
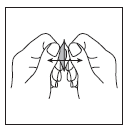
Figure 4
- 4. The tablet should be removed from the packaging with dry hands and placed on the tongue (Figure 5). The tablet will dissolve quickly and can be swallowed without water.
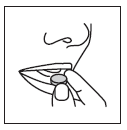
Figure 5
Taking a higher dose of Zolmiles than recommended
If the patient has taken a higher dose of Zolmiles than recommended by their doctor, they should contact their doctor or go to the nearest hospital as soon as possible. They should take Zolmiles with them.
If the patient has swallowed too many oral dispersible tablets, they may experience side effects, including drowsiness.
If the patient has any further questions about the use of this medicine, they should ask their doctor or pharmacist.
4. Possible side effects
Like all medicines, Zolmiles can cause side effects, although not everybody gets them.
Some of the side effects listed below may be part of the migraine attack itself.
If the patient experiences any of the serious side effects listed below, they should stop taking Zolmiles and consult their doctor immediately:
Rare (may affect up to 1 in 1,000 people)
- Allergic reactions including itchy rash (hives) and swelling of the face, lips, tongue and throat.
Very rare (may affect up to 1 in 10,000 people)
- Angina pectoris (chest pain, often caused by exertion), heart attack or coronary artery spasm. Symptoms include chest pain and shortness of breath.
- Spasm of the blood vessels in the intestine, which can cause damage to the intestine. Symptoms include abdominal pain or bloody diarrhea.
Other possible side effects include:
Common (may affect up to 1 in 10 people)
- abnormal sensations, such as tingling in the fingers and toes or skin sensitivity to touch
- feeling of drowsiness, dizziness or feeling of warmth
- headache
- irregular heartbeat
- nausea, vomiting
- abdominal pain
- dry mouth
- swallowing difficulties
- muscle weakness or muscle pain
- feeling of weakness
- heaviness, tension, pain or pressure in the throat, neck, arms and legs or chest.
Uncommon (may affect up to 1 in 100 people)
- very rapid heartbeat
- mild increase in blood pressure
- increased amount of urine passed or frequency of urination.
Very rare (may affect up to 1 in 10,000 people)
- sudden, urgent need to urinate.
Reporting side effects
If the patient experiences any side effects, including any side effects not listed in the leaflet, they should tell their doctor or pharmacist. Side effects can be reported directly to the Department of Drug Adverse Reaction Monitoring, Office for Registration of Medicinal Products, Medical Devices and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, tel.: +48 22 49 21 301, fax: +48 22 49 21 309.
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder. By reporting side effects, more information can be gathered on the safety of the medicine.
5. How to store Zolmiles
The medicine should be stored out of sight and reach of children.
Do not use this medicine after the expiry date stated on the blister pack and carton after: EXP.
The expiry date refers to the last day of the month.
Do not store above 30°C.
Medicines should not be disposed of via wastewater or household waste. The patient should ask their pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the pack and other information
What Zolmiles contains
- The active substance of Zolmiles is zolmitriptan. Zolmiles 2.5 mg oral dispersible tablets contain 2.5 mg of zolmitriptan. Zolmiles 5 mg oral dispersible tablets contain 5 mg of zolmitriptan.
- The other ingredients are: mannitol, calcium silicate, microcrystalline cellulose, aspartame, sodium carboxymethylcellulose (type A), crospovidone (type B), anhydrous colloidal silica, magnesium stearate and orange flavor containing: flavoring substances identical to natural ones, flavor enhancers, natural flavoring substances, maltodextrin (contains glucose), arabic gum, ascorbic acid (E 300), butylhydroxyanisole (E 320).
What Zolmiles looks like and contents of the pack
Zolmiles 2.5 mg oral dispersible tablets are white, round and flat, with a diameter of 7.5 mm.
Zolmiles 5 mg oral dispersible tablets are white, round and flat, with a diameter of 9.5 mm.
Zolmiles is packaged in blister packs with a removable foil cover containing 2, 3, 6 or 12 oral dispersible tablets.
Not all pack sizes may be marketed.
Marketing authorization holder
Actavis Group PTC ehf.
Dalshraun 1
220 Hafnarfjörður
Iceland
Manufacturer
Actavis Ltd.
BLB 016, Bulebel Industrial Estate
Zejtun ZTN 3000
Malta
Teva Pharmaceuticals Polska Sp. z o.o., ul. Emilii Plater 53, 00-113 Warsaw, tel. (22) 345 93 00.
Date of last revision of the leaflet: May 2023
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterActavis Ltd.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ZolmilesDosage form: Tablets, 2.5 mgActive substance: zolmitriptanPrescription requiredDosage form: Tablets, 2.5 mgActive substance: zolmitriptanPrescription requiredDosage form: Tablets, 12.5 mgActive substance: almotriptanManufacturer: Saneca Pharmaceuticals a.s.Prescription required
Alternatives to Zolmiles in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Zolmiles in Spain
Alternative to Zolmiles in Ukraine
Online doctors for Zolmiles
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Zolmiles – subject to medical assessment and local rules.














