ZINERYT 40 mg/ml + 12 mg/ml powder and solvent for cutaneous solution

How to use ZINERYT 40 mg/ml + 12 mg/ml powder and solvent for cutaneous solution
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Zineryt 40 mg/ml + 12 mg/ml powder and solvent for cutaneous solution
Erythromycin/Zinc acetate dihydrate
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What Zineryt is and what it is used for
- What you need to know before you use Zineryt
- How to use Zineryt
- Possible side effects
- Storage of Zineryt
- Contents of the pack and other information
1. What Zineryt is and what it is used for
Zineryt is a medicine that contains two active substances: erythromycin and zinc acetate dihydrate. Erythromycin is an antibiotic belonging to a group of antibiotics called macrolides, and zinc has an anti-inflammatory effect.
Antibiotics are used to treat bacterial infections and are not effective against viral infections. It is important that you follow the instructions regarding dosage, administration interval, and treatment duration as indicated by your doctor. Do not store or reuse this medicine. If you have any leftover antibiotic after completing the treatment, return it to the pharmacy for proper disposal. Do not throw away medicines via wastewater or household waste. |
Zineryt is a topical medicine used for the treatment of mild to moderate common acne in adults.
2. What you need to know before you use Zineryt
Do not use Zineryt
If you are allergic to erythromycin or other macrolide antibiotics, zinc acetate dihydrate, or any of the other components of this medicine (listed in section 6).
Warnings and precautions
Consult your doctor or pharmacist before starting to use this medicine.
This medicine is for external use only.
Avoid contact with the eyes and mucous membranes as it contains ethanol (alcohol). In case of accidental contact with the eyes, rinse with plenty of water and consult your doctor if necessary.
The use of antibiotics may lead to the emergence of microorganisms resistant to erythromycin (loss of antibiotic efficacy), see below "Use of other medicines".
Children
It is not recommended for use in children.
Use of Zineryt with other medicines
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
Especially if you are using or have recently used antibiotics belonging to the group of macrolides or other antibiotics such as lincomycin, clindamycin, and chloramphenicol, as cross-resistance may occur, i.e., it may lead to the emergence of resistant microorganisms.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
Do not use Zineryt if you are pregnant unless clearly necessary.
It should not be administered during breastfeeding.
Driving and using machines
Its use does not affect the ability to drive vehicles or use machines.
3. How to use Zineryt
Follow exactly the administration instructions of this medicine as indicated by your doctor or pharmacist. In case of doubt, consult your doctor or pharmacist again.
For external use only.
Dosage in adults:
Apply the cutaneous solution twice a day (morning and evening) to the face or other affected skin areas, recently washed, (not just on the lesions themselves) until the entire area to be treated is covered.
It is generally recommended to perform the treatment for 10-12 weeks, a period during which, in most cases, a satisfactory improvement is observed. If there has been no improvement or it worsens, the administration of the preparation should be suspended.
Method of administration:
It is applied by tilting the bottle downwards and rubbing the applicator-dispenser over the skin while gently pressing. The amount of liquid obtained depends on the pressure exerted on the skin.
Let it dry. After drying, no stains remain on the skin or clothing that comes into contact with the treated area.
Zineryt should only be used by the same person to avoid contamination.
INSTRUCTIONS FOR THE CORRECT ADMINISTRATION OF THE PREPARATION
PREPARATION
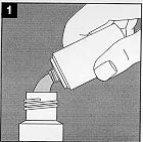
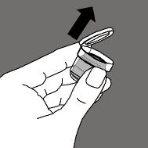
Open the two bottles of Zineryt. |
Pour the contents of the solvent liquid bottle into the bottle containing the powder. (The empty bottle can be discarded). Close the bottle that now contains the liquid and powder. |
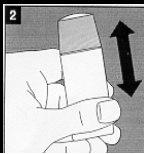
Shake the bottle immediately for one minute. The reconstituted solution is transparent and colorless. |
Remove the cap from the bottle once its contents have been shaken. |
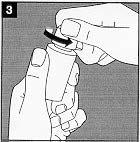
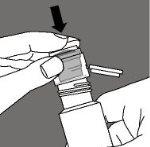
Open the applicator packaging. Do not remove the applicator or touch it with your fingers. |
Turn the applicator packaging over the bottle, pressing firmly until the applicator fits into the bottle mouth. Screw the bottle cap to protect the applicator. |
If you use more Zineryt than you should
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone: 91 562 04 20, indicating the medicine and the amount ingested.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
- Uncommon side effects (may affect up to 1 in 100 people): Burning sensation on the skin, irritation, or mild redness, dryness, itching, and flaking.
- Very rare side effects (may affect up to 1 in 10,000 people): Hypersensitivity reactions (allergic reactions).
Contact a doctor as soon as possible if you experience a severe skin reaction: a red and scaly rash with bumps under the skin and blisters (pustular exanthematous). The frequency of this side effect is considered unknown (cannot be estimated from the available data).
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist, even if it is possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Pharmacovigilance System for Human Use Medicines: www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Zineryt
Keep this medicine out of the sight and reach of children.
- Before reconstitution (powder and solvent): Store below 25°C.
- After reconstitution: Store below 25°C for a maximum of 5 weeks. Write the reconstitution date on the box and on the bottle of the reconstituted solution.
Do not use this medicine after the expiry date which is stated on the packaging after “EXP”. The expiry date is the last day of the month shown.
Medicines should not be disposed of via wastewater or household waste. Return the packaging and any unused medicine to a pharmacy for disposal. If you are unsure, ask your pharmacist how to dispose of the packaging and any unused medicine. This will help protect the environment.
6. Contents of the pack and other information
Composition of Zineryt
- The active substances are erythromycin and zinc acetate dihydrate. Each ml of reconstituted solution contains 40 mg of erythromycin and 12 mg of zinc acetate dihydrate.
- The other components (excipients) are: diisopropyl sebacate and anhydrous ethanol.
Appearance of the product and pack contents
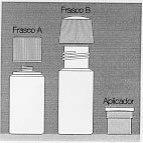
High-density polyethylene bottles with a polypropylene screw cap.
Supplied in a pack containing one bottle with the powder and another bottle with the solvent for reconstitution of 30 ml or 70 ml of solution.
An applicator-dispenser consisting of a spring-loaded device with a sponge is included.
Not all pack sizes may be marketed.
Marketing authorisation holder and manufacturer
Marketing authorisation holder
CHEPLAPHARM Arzneimittel GmbH
Ziegelhof 24
17489 Greifswald
Germany
Local representative
Laboratorios Rubió. S.A.
Industria 29 – Pol. Ind. Comte de Sert
08755 Castellbisbal (Barcelona)
Spain
Manufacturer
LEO Pharma A/S
Industriparken 55
DK-2750 Ballerup
Denmark
Date of last revision of this leaflet: September 2018
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS): http://www.aemps.gob.es/
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ZINERYT 40 mg/ml + 12 mg/ml powder and solvent for cutaneous solutionDosage form: GEL, 1.188 g clindamycin phosphate / gActive substance: clindamycinManufacturer: Chiesi España S.A.U.Prescription requiredDosage form: TOPICAL SOLUTION, 30 mlActive substance: clindamycinManufacturer: Chiesi España S.A.U.Prescription requiredDosage form: EMULSION, 1% clindamycinActive substance: clindamycinManufacturer: Pfizer S.L.Prescription required
Online doctors for ZINERYT 40 mg/ml + 12 mg/ml powder and solvent for cutaneous solution
Discuss questions about ZINERYT 40 mg/ml + 12 mg/ml powder and solvent for cutaneous solution, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions