
Zinerit
Ask a doctor about a prescription for Zinerit

How to use Zinerit
Leaflet attached to the packaging: patient information
Warning! The leaflet should be kept. Information on the immediate packaging in a foreign language.
Zineryt
40 mg/ml + 12 mg/ml, powder and solvent for solution for the skin
Erythromycinum cum zinco
It is necessary to carefully read the contents of the leaflet before using the medicine, as it contains important information for the patient.
- The leaflet should be kept so that it can be re-read if necessary.
- In case of any doubts, you should consult a doctor or pharmacist.
- This medicine has been prescribed for a specific person. It should not be given to others. The medicine may harm another person, even if the symptoms of their disease are the same.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should inform their doctor, pharmacist, or nurse. See section 4.
Table of contents of the leaflet
- 1. What is Zineryt and what is it used for
- 2. Important information before using Zineryt
- 3. How to use Zineryt
- 4. Possible side effects
- 5. How to store Zineryt
- 6. Contents of the packaging and other information
1. What is Zineryt and what is it used for
Zineryt is an anti-acne medicine for use on the skin.
Zineryt contains erythromycin in the form of a complex with zinc acetate. Bacteria sensitive to erythromycin include bacteria such as Staphylococcus epidermidisand Propionibacterium acnes, which often occur in acne. Zinc enhances the effect of erythromycin in local acne treatment.
After drying, Zineryt is invisible on the skin and is therefore cosmetically acceptable.
Indications
Zineryt is indicated for local treatment of moderate to severe acne, in cases where local treatment without antibiotics has been insufficient or not tolerated.
2. Important information before using Zineryt
When not to use Zineryt:
- if the patient is allergic to the active substances or any of the other ingredients of this medicine (listed in section 6);
- if there is hypersensitivity to other macrolide antibiotics.
Warnings and precautions
Before starting treatment with Zineryt, the patient should discuss it with their doctor or pharmacist.
Contact between Zineryt and the eyes or mucous membranes of the nose and mouth should be avoided. The medicine may cause burning and irritation of these areas. If Zineryt comes into contact with the eyes or mucous membranes of the nose and mouth, they should be rinsed thoroughly with water and the doctor should be informed.
Cross-resistance with other macrolide antibiotics and with lincomycin and clindamycin may occur. Mutual cross-hypersensitivity between macrolides may also occur.
Zineryt and other medicines
The patient should inform their doctor or pharmacist about all medicines they are currently taking or have recently taken, as well as any medicines they plan to take.
Interactions between Zineryt and other medicines are not known.
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult their doctor or pharmacist before using this medicine.
Zineryt should not be used during pregnancy, unless the doctor considers it absolutely necessary.
Driving and using machines
There are no data on the effect of Zineryt on the ability to drive and use machines.
However, such an effect is unlikely.
3. How to use Zineryt
This medicine should always be used as directed by the doctor. In case of doubts, the patient should consult their doctor or pharmacist.
Dosage:
Before applying the medicine, the skin surface to be treated should be thoroughly cleaned and dried.
Unless the doctor recommends otherwise, Zineryt should be applied to the affected areas of the skin twice a day, usually for a period of 10 to 12 weeks. A significant therapeutic effect is usually achieved within 12 weeks. If there is no improvement or worsening, the patient should consult their doctor. If the doctor finds that the bacteria are resistant to the medicine, the patient should stop using it for two months.
Method of administration
Zineryt should be applied to the entire face or other affected areas (not just the individual lesion), covering the treated area completely, using about 0.5 ml of solution each time.
To obtain the ready-to-use solution, the patient should follow the instructions in the Instructions for usesection, which appears later in the leaflet.
After preparing the solution, the patient should turn the bottle with the applicator upside down and apply the medicine to the affected skin area, moving the applicator over it. The amount of solution flowing out of the bottle is regulated by increasing or decreasing the pressure of the applicator on the skin.
Instructions for use
Preparing the solution
The packaging contains (see figure):
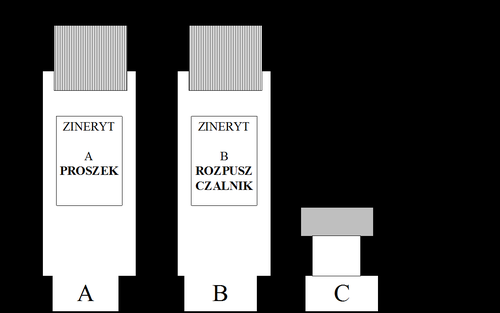
- (A). Bottle with powder for solution
- (B). Bottle with solvent for solution
- (C). Applicator
- 1. After removing the caps, the patient should pour the contents of the solvent bottle (B) into the powder bottle (A).
- 2. The patient should screw the bottle closed, which contains the powder with the solvent, and mix its contents by shaking the bottle for about a minute.
- 3. The patient should remove the cap from the bottle with the resulting solution.
- 4. The patient should place the applicator (C) in the neck of the bottle with the solution, pushing it firmly until it stops.
- 5. The patient should screw the bottle with the applicator closed.
- 6. After preparation, the solution can be used for 8 weeks. The expiration date should be written on the bottle.
Using a higher dose of Zineryt than recommended
The risk of accidental overdose is minimal if the medicine is used as directed.
Accidental ingestion of the entire contents of the Zineryt packaging may cause symptoms of acute ethanol poisoning contained in the medicine.
Missing a dose of Zineryt
If a single dose is missed, treatment can be continued without changes. There is no need to apply an additional amount of medicine to the skin.
In case of any further doubts about using this medicine, the patient should consult their doctor or pharmacist.
4. Possible side effects
Like all medicines, Zineryt can cause side effects, although not everybody gets them.
Uncommon side effects (occurring in 1 to 10 people per 1,000):
itching, redness, skin irritation, burning sensation, stinging, dryness of the skin, peeling of the skin.
Rare side effects (occurring in less than 1 person per 10,000): hypersensitivity.
If the patient experiences a severe skin reaction: red, peeling rash with bumps under the skin and blisters (pustular rash), they should contact their doctor immediately. The frequency of these side effects is unknown (cannot be determined from available data).
Some people may experience other side effects while using Zineryt.
Reporting side effects
If any side effects occur, including any side effects not listed in the leaflet, the patient should inform their doctor, pharmacist, or nurse. Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products
Al. Jerozolimskie 181C
02-222 Warsaw
tel.: + 48 (22) 49 21 301
fax: + 48 (22) 49 21 309
website: https://smz.ezdrowie.gov.pl
Reporting side effects can help gather more information on the safety of the medicine.
5. How to store Zineryt
The medicine should be stored out of sight and reach of children.
Store at a temperature below 25°C.
Store in the original packaging.
The shelf life of the solution after preparation is 8 weeks.
Zineryt should not be used after the expiration date stated on the packaging.
The expiration date refers to the last day of the specified month.
Medicines should not be disposed of via wastewater or household waste. The patient should ask their pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Zineryt contains
1 ml of the ready-to-use solution contains:
- active substances: 40 mg of erythromycin and 12 mg of zinc acetate, in the form of a complex of erythromycin with zinc acetate dihydrate;
- excipients: diisopropyl sebacate, ethanol.
What Zineryt looks like and what the packaging contains
Zineryt is a powder and solvent for solution for the skin.
The packaging of the medicine:
1 bottle with powder and 1 bottle with solvent for preparing 30 ml of solution, and an applicator, in a cardboard box.
The bottle with powder contains the active substances: erythromycin and zinc acetate.
The bottle with solvent contains the excipients: diisopropyl sebacate and ethanol.
For more detailed information, the patient should contact the marketing authorization holder or parallel importer.
Marketing authorization holder in Portugal, the country of export:
Cheplapharm Arzneimittel GmbH, Ziegelhof 24, 17489 Greifswald, Germany
Manufacturer:
LEO Pharma A/S, Industriparken 55, DK-2750 Ballerup, Denmark
Parallel importer:
Delfarma Sp. z o.o., ul. Św. Teresy od Dzieciątka Jezus 111, 91-222 Łódź
Repackaged by:
Delfarma Sp. z o.o., ul. Św. Teresy od Dzieciątka Jezus 111, 91-222 Łódź
Portuguese export license number: 8699207
Parallel import license number: 192/24
Date of leaflet approval: 09.05.2024
[Information about the trademark]
- Country of registration
- Active substance
- Prescription requiredYes
- Marketing authorisation holder (MAH)Cheplapharm Arzneimittel GmbH
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ZineritDosage form: Liquid, (40 mg + 0.25 mg)/gActive substance: erythromycin, combinationsManufacturer: Almirall Hermal GmbHPrescription requiredDosage form: Liquid, (40 mg + 0.25 mg)/gActive substance: erythromycin, combinationsPrescription requiredDosage form: Liquid, (40 mg + 0.25 mg)/gActive substance: erythromycin, combinationsPrescription required
Alternatives to Zinerit in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Zinerit in Ukraina
Alternative to Zinerit in Hiszpania
Online doctors for Zinerit
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Zinerit – subject to medical assessment and local rules.







