
Zinerit

Ask a doctor about a prescription for Zinerit

How to use Zinerit
Leaflet attached to the packaging: patient information
Warning! Keep the leaflet! Information on the immediate packaging in a foreign language.
Zineryt
40 mg/ml + 12 mg/ml, powder and solvent for solution for the skin
Erythromycinum cum zinco
You should carefully read the contents of the leaflet before using the medicine, as it contains important information for the patient.
- You should keep this leaflet, so that you can read it again if you need to.
- If you have any doubts, you should consult a doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor or pharmacist. See section 4.
Table of contents of the leaflet
- 1. What is Zineryt and what is it used for
- 2. Important information before using Zineryt
- 3. How to use Zineryt
- 4. Possible side effects
- 5. How to store Zineryt
- 6. Contents of the packaging and other information
1. What is Zineryt and what is it used for
Zineryt is a medicine for the treatment of acne, for use on the skin.
Zineryt contains erythromycin in the form of a complex with zinc acetate. Bacteria sensitive to erythromycin include Staphylococcus epidermidisand Propionibacterium acnes, which often occur in acne. Zinc enhances the effect of erythromycin in the local treatment of acne.
After drying, Zineryt is invisible on the skin and is therefore cosmetically acceptable.
Indications
Zineryt is indicated for the local treatment of moderate to severe acne, when local treatment without antibiotics has been insufficient or not tolerated.
2. Important information before using Zineryt
When not to use Zineryt:
- if the patient is allergic to the active substances or any of the other ingredients of this medicine (listed in section 6);
- if there is hypersensitivity to other macrolide antibiotics.
Warnings and precautions
Before starting to use Zineryt, you should discuss it with your doctor or pharmacist.
You should avoid contact between Zineryt and the eyes or the mucous membranes of the nose and mouth. The medicine may cause burning and irritation of these areas. If Zineryt comes into contact with the eyes or the mucous membranes of the nose and mouth, they should be rinsed thoroughly with water and the doctor should be informed.
Cross-resistance may occur with other macrolide antibiotics and with lincomycin and clindamycin. Mutual cross-hypersensitivity may also occur between macrolides.
Zineryt and other medicines
You should tell your doctor or pharmacist about all the medicines you are currently taking or have recently taken, as well as any medicines you plan to take.
Interactions between Zineryt and other medicines are not known.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant or are planning to have a baby, you should ask your doctor or pharmacist for advice before using this medicine.
Zineryt should not be used during pregnancy, unless your doctor considers it absolutely necessary.
Driving and using machines
There are no data on the effect of Zineryt on the ability to drive and use machines.
However, such an effect is unlikely.
3. How to use Zineryt
This medicine should always be used in accordance with the doctor's recommendations. If you have any doubts, you should consult your doctor or pharmacist.
Dosage
Before using the medicine, the skin surface to be treated should be thoroughly cleaned and dried.
Unless the doctor recommends otherwise, Zineryt should be applied to the affected areas of the skin twice a day, usually for a period of 10 to 12 weeks. A significant therapeutic effect is usually achieved within 12 weeks. If there is no improvement or if the condition worsens, you should consult your doctor. If the doctor finds that the bacteria are resistant to the medicine, you should stop using it for two months.
Method of administration
Zineryt should be applied to the entire face or other affected areas (not just the individual lesion), until the treated area is completely covered, using about 0.5 ml of solution each time.
To obtain a solution ready for use, you should follow the instructions in the Instructions for use of the medicine, which are included later in this leaflet.
After preparing the solution, you should turn the bottle with the applicator upside down and apply the medicine to the affected area of the skin, moving the applicator over it.
The amount of solution flowing out of the bottle is regulated by increasing or decreasing the pressure of the applicator on the skin.
Instructions for use of the medicine
Preparation of the solution
The packaging contains (see figure):
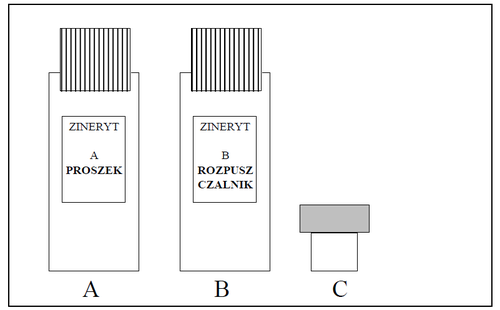
- (A). Bottle with powder for solution
- (B). Bottle with solvent for solution
- (C). Applicator
- 1. After removing the caps, pour the contents of the solvent bottle (B) into the powder bottle (A).
- 2. Close the bottle containing the powder and solvent and mix its contents by shaking the bottle for about one minute.
- 3. Remove the cap from the bottle with the resulting solution.
- 4. Place the applicator (C) in the neck of the bottle with the solution, pushing it firmly until it stops.
- 5. Close the bottle with the applicator.
- 6. After preparation, the solution can be used for 5 weeks. The expiry date should be written on the bottle.
Using a higher dose of Zineryt than recommended
The risk of accidental overdose is minimal if the medicine is used in accordance with the recommendations.
Accidental ingestion of the entire contents of the Zineryt packaging may cause symptoms of acute ethanol poisoning contained in the medicine.
Missing a dose of Zineryt
If a single dose is missed, treatment can be continued without changes. There is no need to apply an additional amount of medicine to the skin.
If you have any further doubts about using this medicine, you should consult your doctor or pharmacist.
4. Possible side effects
Like all medicines, Zineryt can cause side effects, although not everybody gets them.
Uncommon side effects (occurring in 1 to 10 people per 1000):
itching, redness, skin irritation, burning sensation, prickling, dry skin, peeling of the skin.
Rare side effects (occurring in less than 1 person per 10,000):
hypersensitivity.
If the patient experiences a severe skin reaction: red, peeling rash with nodules under the skin and blisters (pustular rash), they should contact their doctor immediately.
The frequency of these side effects is unknown (cannot be determined from the available data).
Some people may experience other side effects while using Zineryt.
Reporting side effects
If you experience any side effects, including any side effects not listed in this leaflet, you should tell your doctor or pharmacist. Side effects can be reported directly to the Department for Monitoring of Adverse Reactions to Medicinal Products, Office for Registration of Medicinal Products, Medical Devices and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, tel.: +48 22 49 21 301, fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl.
Reporting side effects will help to gather more information on the safety of this medicine.
5. How to store Zineryt
The medicine should be kept out of the sight and reach of children.
Store in a temperature below 25°C, in the original packaging.
The shelf life of the solution after preparation is 5 weeks.
You should not use Zineryt after the expiry date stated on the packaging.
The expiry date refers to the last day of the specified month.
Medicines should not be disposed of via wastewater or household waste. You should ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Zineryt contains
1 ml of the ready-to-use solution contains:
- active substances: 40 mg of erythromycin and 12 mg of zinc acetate, in the form of a complex of erythromycin with zinc acetate dihydrate;
- excipients: diisopropyl sebacate, anhydrous ethanol.
What Zineryt looks like and contents of the packaging
Zineryt is a powder and solvent for solution for the skin.
Packaging of the medicine:
1 HDPE bottle with powder and 1 HDPE bottle with solvent for the preparation of 30 ml of solution, and a PP applicator, in a cardboard box.
The bottle with powder contains the active substances: erythromycin and zinc acetate dihydrate.
The bottle with solvent contains the excipients: diisopropyl sebacate and anhydrous ethanol.
For more detailed information, you should contact the marketing authorization holder or the parallel importer.
Marketing authorization holder in Romania, the country of export:
CHEPLAPHARM Arzneimittel GmbH
Ziegelhof 24, 17489 Greifswald, Germany
Manufacturer:
Astellas Pharma Europe B.V.
Hogemaat 2, 7942 JG Meppel, Netherlands
LEO Pharma A/S
Industriparken 55, 2750 Ballerup, Denmark
Parallel importer:
InPharm Sp. z o.o.
ul. Strumykowa 28/11
03-138 Warsaw
Repackaged by:
InPharm Sp. z o.o. Services sp. k.
ul. Chełmżyńska 249
04-458 Warsaw
Authorization number in Romania, the country of export:7452/2015/01
Parallel import authorization number: 125/23
Date of approval of the leaflet: 29.06.2023
[Information about the trademark]
- Country of registration
- Active substance
- Prescription requiredYes
- Marketing authorisation holder (MAH)Cheplapharm Arzneimittel GmbH
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ZineritDosage form: Liquid, (40 mg + 0.25 mg)/gActive substance: erythromycin, combinationsManufacturer: Almirall Hermal GmbHPrescription requiredDosage form: Liquid, (40 mg + 0.25 mg)/gActive substance: erythromycin, combinationsPrescription requiredDosage form: Liquid, (40 mg + 0.25 mg)/gActive substance: erythromycin, combinationsPrescription required
Alternatives to Zinerit in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Zinerit in Ukraina
Alternative to Zinerit in Hiszpania
Online doctors for Zinerit
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Zinerit – subject to medical assessment and local rules.







