
ZILBRYSQ 23 mg, PRE-FILLED SYRINGE SOLUTION

How to use ZILBRYSQ 23 mg, PRE-FILLED SYRINGE SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Zilbrysq 16.6 mg, Solution for Injection in Pre-filled Syringe
Zilbrysq 23 mg, Solution for Injection in Pre-filled Syringe
Zilbrysq 32.4 mg, Solution for Injection in Pre-filled Syringe
zilucoplan
This medicinal product is subject to additional monitoring, which will allow for quick identification of new safety information. You can help by reporting any side effects you may get. The last section of this leaflet includes information on how to report side effects.
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Zilbrysq and what is it used for
- What you need to know before you use Zilbrysq
- How to use Zilbrysq
- Possible side effects
- Storage of Zilbrysq
- Contents of the pack and other information
1. What is Zilbrysq and what is it used for
Zilbrysq contains the active substance zilucoplan. Zilucoplan binds to a protein in the body that produces inflammation, blocking it, known as complement protein C5, which is part of the immune system (the body's natural defenses). By blocking this protein, zilucoplan prevents the immune system from attacking and destroying the connections between nerves and muscles, thus improving the symptoms of the disease.
Zilbrysq is used together with standard treatment to treat adult patients with generalized myasthenia gravis (MG), an autoimmune disease that causes muscle weakness. It is used in adults whose immune system produces antibodies against a protein called acetylcholine receptor, located on muscle cells. In patients with MG, the immune system can attack and damage muscles, which can cause severe muscle weakness, mobility impairment, shortness of breath, extreme fatigue, difficulty swallowing, and functional impairment in performing daily activities.
Zilbrysq may reduce the symptoms of the disease and improve the quality of life.
2. What you need to know before you use Zilbrysq
Do not use Zilbrysq
- if you are allergic to zilucoplan or any of the other ingredients of this medicine (listed in section 6).
- if you have not been vaccinated against meningococcal infection. See section Warnings and precautions.
- if you have any meningococcal infection
Warnings and precautions
Alert about meningococcal and other Neisseria infections
Because Zilbrysq inhibits the body's natural defenses against infections, its use may increase the risk of infections caused by Neisseria meningitidis, such as meningococcal infection (severe infection of the membranes covering the brain and spinal cord and/or infection in the blood) and other infections caused by the Neisseria bacterium, such as gonorrhea.
Consult your doctor before using Zilbrysq to ensure that you are vaccinated against Neisseria meningitidis, a microorganism that causes meningococcal infections, at least 2 weeks before starting treatment. If you cannot be vaccinated 2 weeks in advance, your doctor will prescribe antibiotics to reduce the risk of infection until 2 weeks after receiving the first dose of the vaccine. Make sure your meningococcal vaccinations are up to date.
You should know that vaccination does not always prevent this type of infection.
If you are at risk of getting gonorrhea (a sexually transmitted bacterial infection), ask your doctor before starting this medicine.
Symptoms of meningococcal infection
Due to the importance of quickly identifying and treating meningococcal infections in patients receiving Zilbrysq, you will be given a card to carry with you at all times, which lists specific signs and symptoms of a possible meningococcal infection. It also contains information for healthcare professionals who may not be familiar with Zilbrysq. This card is called: "Patient Alert Card". You will also be given a patient/caregiver guide that contains additional information about Zilbrysq.
Tell your doctor immediately if you have any of these symptoms:
- Headache with additional symptoms such as nausea (feeling sick), vomiting, fever, and neck or back stiffness
- Fever with or without rash
- Sensitivity to light
- Confusion/drowsiness
- Muscle pain with flu-like symptoms
Treatment of meningococcal infections during travel
If you travel to a region from which you cannot contact your doctor or where you cannot temporarily receive medical treatment, your doctor may prescribe an antibiotic against Neisseria meningitidis for you to carry with you. If you have any of the above symptoms, you should take the antibiotic treatment as prescribed. Note that you should see a doctor as soon as possible, even if you feel better after taking the antibiotic treatment.
Children and adolescents
Do not give this medicine to children under 18 years of age. Zilbrysq has not been studied in this age group.
Other medicines and Zilbrysq
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
There is uncertainty about the effects that Zilbrysq may have on the fetus, so do not use this medicine if you are pregnant or think you may be pregnant, unless your doctor specifically recommends it.
It is not known whether Zilbrysq passes into breast milk. There may be a risk to newborns/breastfed infants.
The decision to stop breastfeeding or to stop Zilbrysq treatment should be made taking into account the benefit of breastfeeding for the child and the benefit of treatment for the woman.
Driving and using machines
Zilbrysq is unlikely to affect your ability to drive or use machines.
Zilbrysq contains sodium
This medicine contains less than 1 mmol (23 mg) of sodium per pre-filled syringe, which is essentially "sodium-free".
3. How to use Zilbrysq
At least 2 weeks before starting treatment with Zilbrysq, your doctor will give you a vaccine against meningococcal infection if you have not received it before or if your vaccination needs to be renewed. If you cannot be vaccinated at least 2 weeks before starting treatment with Zilbrysq, your doctor will prescribe antibiotics to reduce the risk of infection until 2 weeks after receiving the first dose of the vaccine.
Before starting treatment, you should also consult your doctor if you need any other vaccine.
After proper training, your doctor will allow you to inject Zilbrysq yourself. Follow exactly the administration instructions of this medicine indicated by your doctor. If in doubt, consult your doctor again.
The dose you receive will depend on your body weight. Always administer your daily dose at about the same time of day.
The following table indicates the total daily dose of Zilbrysq according to your body weight:
Body weight | Dose | Number of pre-filled syringes by color |
<56 kg | 16.6 mg | 1 (FUCHSIA) |
≥56 to <77 kg | 23 mg | 1 (ORANGE) |
≥77 kg | 32.4 mg | 1 (DARK BLUE) |
How to administer Zilbrysq
You and your doctor or nurse will decide if you can inject this medicine yourself. Do not inject yourself with this medicine unless a healthcare professional has taught you how to do it. Another person can also give you the injections after receiving training.
Zilbrysq will be administered as a subcutaneous injection (an injection under the skin) once a day. It can be injected into the abdomen, the front of the thighs, or the outer aspect of the upper arm. Injections into the outer aspect of the upper arms should only be given by another person. The injection site should be changed each time, avoiding areas where the skin is sensitive, bruised, red, or hardened, or where there are scars or stretch marks.
It is important that you read the instructions for use at the end of the leaflet for detailed information on how to use Zilbrysq.
If you use more Zilbrysq than you should
If you suspect that you have accidentally received a higher dose of Zilbrysq than prescribed, ask your doctor for advice.
If you forget to use Zilbrysq
If you have not injected the dose at the usual time or have forgotten a dose, inject it as soon as you remember and then continue with the administration at the usual time the next day. Do not administer more than one dose per day.
If you stop using Zilbrysq
Stopping or interrupting treatment with Zilbrysq may cause your symptoms to return. Talk to your doctor before stopping Zilbrysq. Your doctor will explain the possible side effects and risks. Your doctor may also want to monitor you closely.
If you have any other questions about the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Very common(may affect more than 1 in 10 people)
- Reactions at the injection site, such as bruising, pain, itching, and lump formation.
- Nasal and throat infection.
Common(may affect up to 1 in 10 people)
- Diarrhea
- Elevation of pancreatic enzymes (amylase, lipase) in blood tests.
- Morphea (a condition that causes localized discoloration and hardening of skin areas)
Uncommon(may affect up to 1 in 100 people)
- Elevation of eosinophils (a type of white blood cell) in blood tests.
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Zilbrysq
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label of the pre-filled syringe and on the carton after EXP/CAD. The expiry date is the last day of the month stated.
Store in a refrigerator (between 2°C and 8°C).
Do not freeze.
Keep the pre-filled syringe in the outer packaging to protect it from light.
You can store the Zilbrysq pre-filled syringe at room temperature in its original carton at a maximum temperature of 30°C for a single period of up to 3 months. Once Zilbrysq is removed from the refrigerator, it must not be put back. The product should be discarded if it is not used within 3 months, or if it reaches the expiry date (whichever comes first).
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Contents and Additional Information
Zilbrysq Composition
- The active ingredient is: zilucoplan.
- The other components are: sodium dihydrogen phosphate monohydrate, disodium phosphate (anhydrous), sodium chloride, water for injectables. (See section 2, "Zilbrysq contains sodium").
Appearance of Zilbrysq and Container Contents
Zilbrysq is an injectable solution in a pre-filled syringe (injectable) and is between transparent and slightly opalescent and colorless, with no visible particles.
Zilbrysq 16.6 mg, injectable solution in pre-filled syringe
Each pre-filled syringe with a pink plunger contains zilucoplan sodium equivalent to 16.6 mg of zilucoplan in 0.416 ml.
Zilbrysq 23 mg, injectable solution in pre-filled syringe
Each pre-filled syringe with an orange plunger contains zilucoplan sodium equivalent to 23 mg of zilucoplan in 0.574 ml.
Zilbrysq 32.4 mg, injectable solution in pre-filled syringe
Each pre-filled syringe with a dark blue plunger contains zilucoplan sodium equivalent to 32.4 mg of zilucoplan in 0.810 ml.
Container size: 7 pre-filled syringes for 16.6 mg, 23 mg, and 32.4 mg of injectable solution.
Multipack of 28 (4 packs of 7) pre-filled syringes.
Only some pack sizes may be marketed.
Marketing Authorization Holder
UCB Pharma S.A., Allée de la Recherche 60, B-1070 Brussels, Belgium
Manufacturer
UCB Pharma S.A., Chemin du Foriest, B-1420 Braine-l’Alleud, Belgium.
You can request more information about this medicinal product by contacting the local representative of the marketing authorization holder:
België/Belgique/Belgien UCB Pharma S.A./NV Tel: + 32 / (0)2 559 92 00 | Lietuva UAB Medfiles Tel: + 370 5 246 16 40 |
| Luxembourg/Luxemburg UCB Pharma SA/NV Tel: + 32 / (0)2 559 92 00 (Belgique/Belgien) |
Ceská republika UCB s.r.o. Tel: + 420 221 773 411 | Magyarország UCB Magyarország Kft. Tel.: + 36-(1) 391 0060 |
Danmark UCB Nordic A/S Tlf.: + 45 / 32 46 24 00 | Malta Pharmasud Ltd. Tel: + 356 / 21 37 64 36 |
Deutschland UCB Pharma GmbH Tel: + 49 /(0) 2173 48 4848 | Nederland UCB Pharma B.V. Tel: + 31 / (0)76-573 11 40 |
Eesti OÜ Medfiles Tel: + 372 730 5415 | Norge UCB Nordic A/S Tlf: + 47 / 67 16 5880 |
Ελλάδα UCB Α.Ε. Τηλ: + 30 / 2109974000 | Österreich UCB Pharma GmbH Tel: + 43-(0)1 291 80 00 |
España UCB Pharma, S.A. Tel: + 34 / 91 570 34 44 | Polska UCB Pharma Sp. z o.o. / VEDIM Sp. z o.o. Tel: + 48 22 696 99 20 |
France UCB Pharma S.A. Tél: + 33 / (0)1 47 29 44 35 | Portugal UCB Pharma (Produtos Farmacêuticos), Lda Tel: + 351 21 302 5300 |
Hrvatska Medis Adria d.o.o. Tel: +385 (0) 1 230 34 46 | România UCB Pharma Romania S.R.L. Tel: + 40 21 300 29 04 |
Ireland UCB (Pharma) Ireland Ltd. Tel: + 353 / (0)1-46 37 395 | Slovenija Medis, d.o.o. Tel: + 386 1 589 69 00 |
Ísland Vistor hf. Simi: + 354 535 7000 | Slovenská republika UCB s.r.o., organizačná zložka Tel: + 421 (0) 2 5920 2020 |
Italia UCB Pharma S.p.A. Tel: + 39 / 02 300 791 | Suomi/Finland UCB Pharma Oy Finland Puh/Tel: + 358 9 2514 4221 |
Κύπρος Lifepharma (Z.A.M.) Ltd Τηλ: + 357 22 056300 | Sverige UCB Nordic A/S Tel: + 46 / (0) 40 294 900 |
Latvija Medfiles SIA Tel: + 371 67 370 250 | United Kingdom (Northern Ireland) UCB (Pharma) Ireland Ltd. Tel : + 353 / (0)1-46 37 395 |
Date of Last Revision of this Leaflet:
Other Sources of Information
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu. There are also links to other websites on rare diseases and orphan medicines.
Zilbrysq Administration Instructions, Solution for Injection in Pre-filled Syringe
Read ALL the Following Instructions Before Using Zilbrysq
Before Use
After Use
Important Information:
- Your healthcare professional should teach you how to prepare and inject Zilbrysq correctly before you do it for the first time.
- Call your healthcare professional if you or your caregiver have any questions about how to inject Zilbrysq correctly.
Do Not Use this Medicinal Product and Return it to the Pharmacy if:
- The pre-filled syringe has been dropped.
Follow these Steps Each Time you Use Zilbrysq
- Step 1: Prepare the Injection
- If the pre-filled syringes are stored in the refrigerator, for a more comfortable injection: Remove 1 pre-filled syringe of Zilbrysq from the refrigerator and let it sit on a clean, flat surface at room temperature for 30 to 45 minutes before injecting. Do not warm it up in any other way. Put the rest of the box back in the refrigerator and continue with the next step.
If the pre-filled syringes are stored at room temperature:Remove 1 pre-filled syringe of Zilbrysq from the box. The other syringes in the box should not be stored in the refrigerator once they have been stored at room temperature.
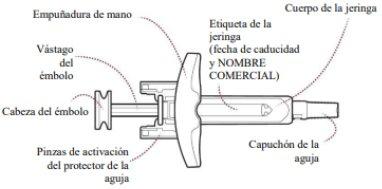
When removing a syringe from the outer box, hold the syringe by the body (Figure A). Do nottouch the plunger rod or the needle cap. Do nottouch the needle shield activation clips at any time, as this may cause premature activation of the needle shield.
Figure A
- Place the Following Items on a Clean, Flat, and Well-lit Surface, such as a Table:
- 1 pre-filled syringe of Zilbrysq
- 1 alcohol swab (not included)
- 1 cotton ball or gauze (not included)
- 1 adhesive dressing (not included)
- 1 puncture-proof container (not included). See Step 4 for instructions on how to dispose of the empty syringe.
- Examine the Pre-filled Syringe.
- Check if the pre-filled syringe is damaged (Figure "Before Use").
- Do notuse the pre-filled syringe if any part of it appears to be cracked, leaking, or broken.
- Do notuse it if the needle cap is cracked, broken, missing, or not securely attached to the pre-filled syringe.
- Do not remove the needle cap from the pre-filled syringe until you are ready to inject.
- Do not use it if the liquid has been frozen at any time (even if it has been thawed)
- Check the expiration date on the syringe label.
- Examine the medicine inside the pre-filled syringe. The medicine should be between transparent and slightly opalescent and colorless. It is normal to have air bubbles in the syringe. Do notuse it if the medicine is cloudy, has changed color, or contains floating particles.
- Check the dose shown on the label. Do notuse it if the dose does not match the one prescribed for you.
- Step 2: Choose the Injection Site and Prepare it.
- Choose the Injection Site.
Choose an injection site from the following areas (Figure B):
- The abdomen (except for the area within 5 cm of the navel).
- The front of the thighs.
- The outer aspect of the upper arms.
Figure B
- Abdomen and thighs.
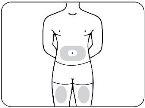
- The outer aspect of the upper arms (only if someone else is giving you the injection).
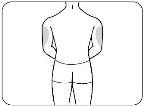
Choose a different site for each injection. If you want to use the same injection site, make sure it is at least 2.5 cm away from the site you used last time.
Do notinject Zilbrysq into an area that is sensitive, red, bruised, hardened, or scarred.
- Wash Your Handswith water and soap, and dry them with a clean towel.
- Prepare the Skin
- Clean the injection site with an alcohol swab.
- Let the skin dry for 10 seconds before injecting.
- Do nottouch the injection site again before injecting.
- Step 3: Inject Zilbrysq
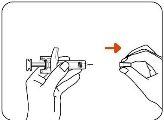
- Remove the Needle Cap
Hold the Zilbrysq pre-filled syringe by the body with one hand and pull the needle cap straight off with the other hand (Figure C).
Dispose of the Needle Cap in the Household Trash or a Puncture-proof Container (see Step 4).
- Do nottouch the needle or let it touch anything.
- To avoid injury, do notattempt to replace the needle cap at any time.
- Do nottry to remove air bubbles from the syringe. Air bubbles will not affect your dose and will not harm you. It is normal. You can proceed with the injection.
Figure C
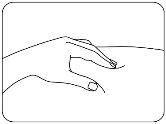
- Pinch the Injection Site.
With the other hand, pinch the clean skin firmly and hold it in place (Figure D).
Figure D
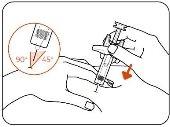
- Insert the Needle.
Insert the entire needle into the pinched skin at an angle of 45° to 90° (Figure E).
- Do notpull the plunger at any time, as this may break the syringe.
- Do nottouch the needle shield activation clips.
Figure E
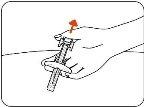
- Release the Skin.
When the needle is fully inserted, hold the pre-filled syringe in place and release the pinched skin (Figure F).
- Do notreinsert the needle into the skin if the needle comes out when releasing the skin, as the needle may bend or break, causing tissue damage. If this happens, safely dispose of the syringe in a puncture-proof container and take a new syringe for injection.
Figure F
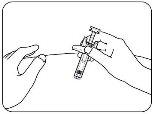
- Inject the Medicine.
Push the plunger all the way down while holding the wings to inject all the medicine (Figure G). The medicine will have been fully injected when you can no longer push the plunger head.
Figure G
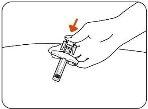
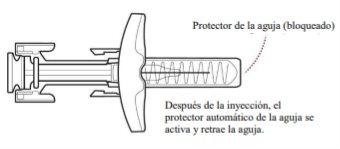
- Release the Plunger.
Slowly release the plunger by lifting your thumb. When the injection is complete, the needle shield will cover the needle, and you may hear a click (Figure H).
Figure H
- Examine the Injection Site.
Press a cotton ball or gauze over the injection site and hold it for 10 seconds.
Do notrub the injection site. It may bleed a little; this is normal. Apply an adhesive dressing if necessary.
Step 4: Dispose of the Used Syringe in a Puncture-proof Container Immediately.
Always keep the puncture-proof container out of the reach of children.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ZILBRYSQ 23 mg, PRE-FILLED SYRINGE SOLUTIONDosage form: INJECTABLE, 16.6 mgActive substance: zilucoplanManufacturer: Ucb PharmaPrescription requiredDosage form: INJECTABLE, 32.4 mgActive substance: zilucoplanManufacturer: Ucb PharmaPrescription requiredDosage form: INJECTABLE PERFUSION, 1080 mgActive substance: pegcetacoplanManufacturer: Swedish Orphan Biovitrum Ab (Publ)Prescription required
Online doctors for ZILBRYSQ 23 mg, PRE-FILLED SYRINGE SOLUTION
Discuss questions about ZILBRYSQ 23 mg, PRE-FILLED SYRINGE SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions







