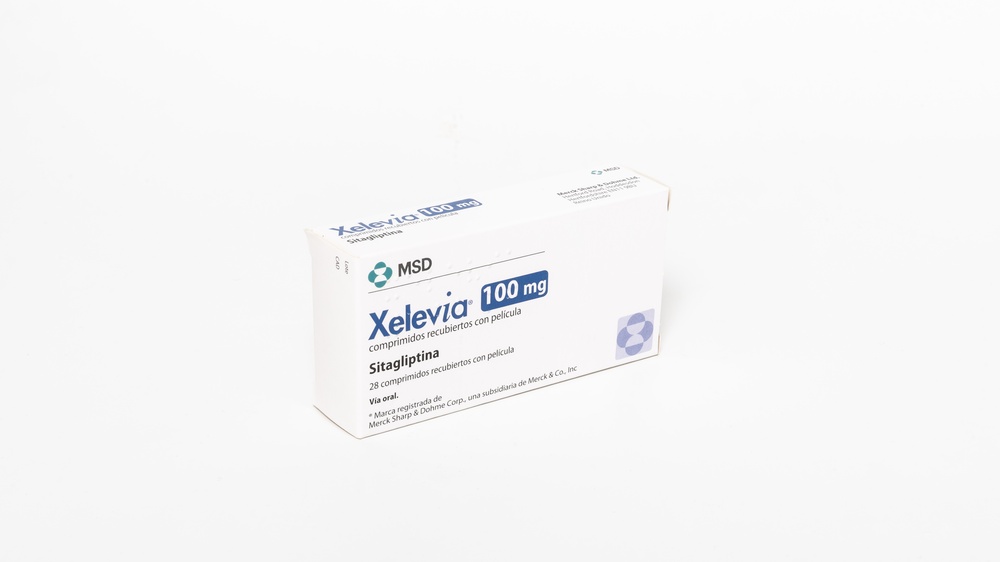
XELEVIA 100 mg FILM-COATED TABLETS

How to use XELEVIA 100 mg FILM-COATED TABLETS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
- Introduction
- What is Xelevia and what is it used for
- What you need to know before you take Xelevia
- How to take Xelevia
- Follow exactly the instructions of your doctor for taking this medicine. If you are not sure, ask your doctor or pharmacist again.
- Possible side effects
- Storing Xelevia
- Contents of the pack and further information
Introduction
Package Leaflet: Information for the User
Xelevia 25mg film-coated tablets
Xelevia 50mg film-coated tablets
Xelevia 100mg film-coated tablets
sitagliptin
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack and other information
- What is Xelevia and what is it used for
- What you need to know before you take Xelevia
- How to take Xelevia
- Possible side effects
- Storing Xelevia
- Contents of the pack and further information
1. What is Xelevia and what is it used for
Xelevia contains the active substance sitagliptin, which belongs to a class of medicines called DPP-4 inhibitors (dipeptidyl peptidase-4 inhibitors) that reduce blood sugar levels in adult patients with type 2 diabetes.
This medicine helps to increase the amount of insulin produced after a meal and decreases the amount of sugar produced by the body.
Your doctor has prescribed this medicine to help you reduce your blood sugar level, which is too high due to your type 2 diabetes. This medicine can be used alone or in combination with other medicines (insulin, metformin, sulfonylureas or glitazones) that reduce blood sugar levels, and that you may already be taking for your diabetes, together with a diet and exercise program.
What is type 2 diabetes?
Type 2 diabetes is a condition where your body does not produce enough insulin, and the insulin that it does produce does not work as well as it should. Your body may also produce too much sugar. When this happens, sugar (glucose) builds up in the blood. This can cause serious medical problems, such as heart disease, kidney disease, blindness, and amputation.
2. What you need to know before you take Xelevia
Do not take Xelevia
- if you are allergic to sitagliptin or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
There have been reports of pancreatitis (inflammation of the pancreas) in patients taking Xelevia (see section 4).
If you notice blisters on your skin, it may be a sign of a disease called bullous pemphigoid. Your doctor may ask you to stop taking Xelevia.
Tell your doctor if you have or have had:
- a disease of the pancreas (such as pancreatitis)
- gallstones, alcohol dependence, or very high levels of triglycerides (a type of fat) in the blood. These medical conditions may increase your risk of developing pancreatitis (see section 4)
- type 1 diabetes
- diabetic ketoacidosis (a complication of diabetes that causes high blood sugar levels, rapid weight loss, nausea, or vomiting)
- any current or past kidney problems
- an allergic reaction to Xelevia (see section 4)
It is unlikely that this medicine will cause low blood sugar because it does not work when your blood sugar levels are low. However, when this medicine is used in combination with a medicine that contains a sulfonylurea or with insulin, low blood sugar (hypoglycemia) may occur. Your doctor may reduce the dose of your sulfonylurea or insulin medicine.
Children and adolescents
Children and adolescents under 18 years of age should not use this medicine. It is not effective in children and adolescents aged 10 to 17 years. It is not known if this medicine is safe and effective when used in children under 10 years of age.
Other medicines and Xelevia
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines.
In particular, tell your doctor if you are taking digoxin (a medicine used to treat irregular heartbeat and other heart problems). Your doctor may need to check the level of digoxin in your blood if you are taking Xelevia.
Pregnancy and breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
This medicine should not be taken during pregnancy.
It is not known if this medicine passes into breast milk. You should not take this medicine if you are breast-feeding or planning to breast-feed.
Driving and using machines
This medicine has no or negligible influence on the ability to drive and use machines. However, dizziness and somnolence have been reported, which may affect your ability to drive or use machines.
Also, taking this medicine with sulfonylureas or with insulin may cause low blood sugar (hypoglycemia), which may affect your ability to drive or use machines or work without a safe support.
Xelevia contains sodium
This medicine contains less than 23 mg of sodium (1 mmol) per tablet; this is essentially "sodium-free".
3. How to take Xelevia
Follow exactly the instructions of your doctor for taking this medicine. If you are not sure, ask your doctor or pharmacist again.
The recommended dose is:
- one 100 mg film-coated tablet
- once a day
- by mouth
If you have kidney problems, your doctor may prescribe a lower dose (such as 25 mg or 50 mg).
You can take this medicine with or without food and drinks.
Your doctor may prescribe this medicine alone or with other medicines that also reduce blood sugar levels, and that you may already be taking for your diabetes, together with a diet and exercise program.
Diet and exercise may help your body use sugar better. It is important that you follow the diet and exercise recommended by your doctor while taking Xelevia.
If you take more Xelevia than you should
If you take more of this medicine than you should, contact your doctor immediately.
If you forget to take Xelevia
If you forget to take a dose, take it as soon as you remember. If you do not remember until the time for the next dose, then skip the missed dose and continue with your normal routine. Do not take a double dose of this medicine.
If you stop taking Xelevia
Keep taking this medicine while your doctor recommends it to help keep your blood sugar levels under control. You should not stop taking this medicine without first talking to your doctor.
If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
STOP taking Xelevia and contact a doctor immediatelyif you notice any of the following serious side effects:
- Severe and persistent pain in the abdomen (stomach area) that may radiate to the back, with or without nausea and vomiting, as these may be signs of pancreatitis.
If you have a severe allergic reaction (frequency not known), including skin rash, hives, blisters on the skin/peeling of the skin, and swelling of the face, lips, tongue, and throat that may cause difficulty breathing or swallowing, stop taking this medicine and contact your doctor immediately. Your doctor will prescribe a medicine to treat the allergic reaction and will change your diabetes treatment.
Some patients reported the following side effects after adding sitagliptin to their treatment with metformin:
Common (may affect up to 1 in 10 people): low blood sugar, nausea, flatulence, vomiting
Uncommon (may affect up to 1 in 100 people): stomach pain, diarrhea, constipation, somnolence.
Some patients reported different types of stomach discomfort when starting the combination of sitagliptin and metformin together (frequency classified as common).
Some patients reported the following side effects while taking sitagliptin in combination with a sulfonylurea and metformin:
Very common (may affect more than 1 in 10 people): low blood sugar
Common: constipation
Some patients reported the following side effects while taking sitagliptin and pioglitazone:
Common: flatulence, swelling of hands or feet
Some patients reported the following side effects while taking sitagliptin and pioglitazone and metformin:
Common: swelling of hands or feet
Some patients reported the following side effects while taking sitagliptin in combination with insulin (with or without metformin):
Common: flu
Uncommon: dry mouth
Some patients reported the following side effects while taking sitagliptin alone during clinical trials, or during use after approval alone and/or with other diabetes medicines:
Common: low blood sugar, headache, infection of the upper respiratory tract, nasal congestion or runny nose, and sore throat, osteoarthritis, pain in the arm or leg
Uncommon: dizziness, constipation, itching
Rare: reduced number of platelets
Frequency not known: kidney problems (which may require dialysis), vomiting, joint pain, muscle pain, back pain, interstitial lung disease, bullous pemphigoid (a type of blistering skin condition)
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storing Xelevia
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the blister and carton after EXP. The expiry date refers to the last day of the month shown.
Store below 25°C.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Contents of the pack and further information
What Xelevia contains
- The active substance is sitagliptin:
- Each Xelevia 25 mg film-coated tablet contains sitagliptin phosphate monohydrate equivalent to 25 mg of sitagliptin.
- Each Xelevia 50 mg film-coated tablet contains sitagliptin phosphate monohydrate equivalent to 50 mg of sitagliptin.
- Each Xelevia 100 mg film-coated tablet contains sitagliptin phosphate monohydrate equivalent to 100 mg of sitagliptin.
- The other ingredients are:
- Tablet core: microcrystalline cellulose (E460), anhydrous calcium hydrogen phosphate (E341), sodium carmellose (E468), magnesium stearate (E470b), sodium stearyl fumarate, and propyl gallate.
- Film coating: polyvinyl alcohol, macrogol 3350, talc (E553b), titanium dioxide (E171), red iron oxide (E172), and yellow iron oxide (E172).
Appearance and packaging
- Xelevia 25 mg film-coated tablets are round, pink, with "221" on one side.
- Xelevia 50 mg film-coated tablets are round, light beige, with "112" on one side.
- Xelevia 100 mg film-coated tablets are round, beige, with "277" on one side.
Opaque blisters (PVC/PE/PVDC and aluminum). Packs of 14, 28, 30, 56, 84, 90, or 98 film-coated tablets and 50 x 1 film-coated tablets in unit dose blisters.
Not all pack sizes may be marketed.
Marketing Authorisation Holder and Manufacturer
Merck Sharp & Dohme B.V.
Waarderweg 39
2031 BN Haarlem
Netherlands
For further information about this medicine, contact your local representative of the Marketing Authorisation Holder:
Belgium MSD Belgium Tel: +32 (0)27766211 | Lithuania UAB Merck Sharp & Dohme Tel: +370 5 278 02 47 |
| Luxembourg MSD Belgium Tel: +32 (0)27766211 |
Czech Republic Merck Sharp & Dohme s.r.o. Tel: +420 233 010 111 | Hungary MSD Pharma Hungary Kft. Tel: +36 1 8885300 |
Denmark MSD Danmark ApS Tel: +45 4482 4000 | Malta Merck Sharp & Dohme Cyprus Limited Tel: 8007 4433 (+356 99917558) |
Germany BERLIN-CHEMIE AG Tel: +49 (0) 30 67070 | Netherlands Merck Sharp & Dohme B.V. Tel: 0800 9999000 (+31 23 5153153) |
Estonia Merck Sharp & Dohme OÜ Tel: +372 6144 200 | Norway MSD (Norge) AS Tel: +47 32 20 73 00 |
Greece MSD Α.Φ.Ε.Ε. Tel: +30 210 98 97 300 | Austria Merck Sharp & Dohme Ges.m.b.H. Tel: +43 (0) 1 26 044 |
Spain Merck Sharp & Dohme de España, S.A. Tel: +34 91 321 06 00 | Poland MSD Polska Sp. z o.o. Tel: +48 22 549 51 00 |
France MSD France Tel: +33 (0)1 80 46 40 40 | Portugal Merck Sharp & Dohme, Lda Tel: +351 21 4465700 |
Croatia Merck Sharp & Dohme d.o.o. Tel: +385 1 66 11 333 | Romania Merck Sharp & Dohme Romania S.R.L. Tel: +4021 529 29 00 |
Ireland Merck Sharp & Dohme Ireland (Human Health) Limited Tel: +353 (0)1 2998700 | Slovenia Merck Sharp & Dohme, inovativna zdravila d.o.o. Tel: +386 1 5204 201 |
Iceland Vistor hf. Tel: +354 535 7000 | Slovakia Merck Sharp & Dohme, s. r. o. Tel: +421 2 58282010 |
Italy Neopharmed Gentili S.p.A. Tel: +39 02891321 | Finland MSD Finland Oy Tel: +358 (0)9 804 650 |
Cyprus Merck Sharp & Dohme Cyprus Limited Tel: 80000 673 (+357 22866700) | Sweden Merck Sharp & Dohme (Sweden) AB Tel: +46 77 5700488 |
Latvia SIA Merck Sharp & Dohme Latvija Tel: +371 67025300 | United Kingdom (Northern Ireland) Merck Sharp & Dohme Ireland (Human Health) Limited Tel: +353 (0)1 2998700 |
Date of last revision of this leaflet:{MM/AAAA}.
Detailed information on this medicine is available on the European Medicines Agency web site: http://www.ema.europa.eu.
- Country of registration
- Average pharmacy price26.52 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to XELEVIA 100 mg FILM-COATED TABLETSDosage form: TABLET, 100 mgActive substance: sitagliptinManufacturer: Laboratorios Alter S.A.Prescription requiredDosage form: TABLET, 25 mgActive substance: sitagliptinManufacturer: Laboratorios Alter S.A.Prescription requiredDosage form: TABLET, 50 mgActive substance: sitagliptinManufacturer: Laboratorios Alter S.A.Prescription required
Online doctors for XELEVIA 100 mg FILM-COATED TABLETS
Discuss questions about XELEVIA 100 mg FILM-COATED TABLETS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions