
WYNZORA 50 micrograms/g + 0.5 mg/g Cream

How to use WYNZORA 50 micrograms/g + 0.5 mg/g Cream
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Wynzora 50micrograms/g + 0.5mg/g cream
calcipotriol/betamethasone
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Wynzora and what is it used for
- What you need to know before you use Wynzora
- How to use Wynzora
- Possible side effects
- Storing Wynzora
- Contents of the pack and other information
1. What is Wynzora and what is it used for
This medicine is used on the skin to treat mild to moderate plaque psoriasis, including scalp psoriasis, in adults. Psoriasis is caused because your skin cells are produced too quickly. This leads to redness, scaling, and thickening of the skin.
This medicine contains calcipotriol and betamethasone. Calcipotriol helps to bring the rate of skin cell growth back to normal and betamethasone acts by reducing inflammation.
2. What you need to know before you use Wynzora
Do not use Wynzora
- if you are allergic to calcipotriol, betamethasone, or any of the other ingredients of this medicine (listed in section 6)
- if you have problems with your calcium levels (consult your doctor)
- if you suffer from other types of psoriasis, such as erythrodermic, exfoliative, or pustular psoriasis (consult your doctor).
Since this medicine contains a potent corticosteroid, DO NOT use it on skin affected by:
- skin infections caused by viruses (e.g., cold sores or chickenpox)
- skin infections caused by fungi (e.g., athlete's foot or ringworm)
- bacterial skin infections
- parasitic skin infections (e.g., scabies)
- tuberculosis (TB)
- perioral dermatitis (red rash around the mouth)
- thin skin, fragile veins, stretch marks
- ichthyosis (dry skin with fish-like scales)
- acne (pimples)
- rosacea (intense redness or flushing of the skin on the face)
- ulcers or cracked skin.
Warnings and precautions
Consult your doctor, pharmacist, or nurse before you start usingWynzora:
- if you have diabetes mellitus (diabetes), as the level of sugar/glucose in your blood may be affected by the corticosteroid
- if you are using other medicines that contain corticosteroids, as you may experience side effects
- if you have a certain type of psoriasis called guttate psoriasis.
Consult your doctor, pharmacist, or nurse during treatment:
- if you have used this medicine for a long time and plan to stop (as there is a risk that your psoriasis may worsen or flare up if corticosteroids are stopped suddenly)
- if a skin infection appears, as you may need to interrupt treatment
- if your blood calcium level changes (see section 4 for more information)
- if you experience blurred vision or other visual disturbances.
Special precautions
- Avoid using it on more than 30% of your body or more than 15 grams per day;
- avoid using it with shower caps, bandages, or dressings, as this increases the absorption of the corticosteroid;
- avoid using it on large areas of damaged skin, on mucous membranes, or in skin folds (groin, armpits, under the breasts) as this increases the absorption of the corticosteroid;
- avoid using it on the face or genitals (sex organs), as they are very sensitive to corticosteroids;
- avoid excessive sunbathing, excessive use of sunbeds, and other forms of light treatment or exposure.
Children and adolescents
This medicine is not recommended for use in children and adolescents under 18 years of age.
Other medicines and Wynzora
Tell your doctor, pharmacist, or nurse if you are using, have recently used, or might use any other medicines.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
Pregnancy
This medicine should only be used during pregnancy if your doctor advises it.
Breastfeeding
If your doctor agrees that you can continue breastfeeding, be careful and do not apply this medicine to the breast area.
Driving and using machines
This medicine has no or negligible influence on your ability to drive or use machines.
Wynzora contains butylhydroxyanisole (E320) and macrogolglycerol hydroxystearate
This medicine may cause local skin reactions (such as contact dermatitis) or eye and mucous membrane irritation because it contains butylhydroxyanisole (E320) and macrogolglycerol hydroxystearate.
3. How to use Wynzora
Follow the instructions for administration of this medicine exactly as advised by your doctor. If you are unsure, consult your doctor or pharmacist again.
This medicine is for cutaneous use (on the skin).
Instructions for proper use
When using on the body
- Use only on skin with psoriasis and not on skin without psoriasis.
- Apply the cream to a clean finger or directly to the affected psoriasis area.
- Apply the cream to the affected area with your fingertips and spread it out in a thin layer.
- Do not bandage, cover, or wrap the treated skin area.
- Wash your hands well after using this medicine. This will prevent you from accidentally spreading the cream to other parts of your body (especially the face, mouth, and eyes).
- Do not worry if you accidentally apply a small amount of cream to normal skin near the psoriasis, but clean it off if it spreads too much.
- In order to achieve the best effect, it is recommended not to shower or bathe immediately after applying this medicine. It is recommended to wait 8 hours between application and showering to avoid removing the cream.
- After applying the cream, avoid contact with textiles as they may stain easily with the grease it contains (e.g., silk).
When using on the scalp
- Before applying this medicine to the scalp, comb your hair to remove any loose scales.
- It may be helpful to part your hair before using this medicine.
- You do not need to wash your hair before applying this medicine.
- Apply cream to the fingertip, apply directly to areas where you can feel the plaques, and massage well into a thin layer.
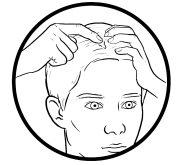
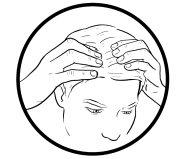
- In order to achieve the best effect, it is recommended not to wash your hair after applying this medicine.
- It is recommended to wait 8 hours between application and showering to avoid removing the cream.
Treatment duration
- Use the cream once a day. It may be more convenient to use the cream at night.
- The initial treatment period is normally 8 weeks.
- Your doctor may decide on a different treatment period.
- Your doctor may decide if you need to repeat the treatment.
- Do not use more than 15 grams in one day.
If you use other medicines that contain calcipotriol, the total amount of these medicines should not exceed 15 grams per day, and the treated area should not exceed 30% of the total body surface area.
What to expect when using Wynzora
Most patients notice results after 1 week of treatment, even if the psoriasis has not completely cleared at that time.
If you use more Wynzora than you should
Contact your doctor if you have used more than 15 grams in one day.
Overuse of this medicine can also cause a calcium problem in the blood, which usually returns to normal when treatment is stopped. Your doctor may need you to have blood tests to check that overuse of the cream does not cause you any problems with calcium in the blood.
Prolonged overuse can also cause your adrenal glands to stop working properly (the adrenal glands are located above the kidneys and produce hormones).
If you use more Wynzora than you should, contact your doctor or pharmacist immediately or call the Toxicology Information Service, phone 915 620 420, stating the medicine and the amount used.
If you forget to use Wynzora
Do not take a double dose to make up for forgotten doses.
If you stop using Wynzora
You should stop using this medicine as advised by your doctor. It may be necessary to stop using this medicine gradually, especially if you have used it for a long time.
If you have any other questions about using this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Side effects that have been seen with Wynzora:
Uncommon (may affect up to 1 in 100 people):
- Inflammation or swelling of the hair root at the application site (folliculitis)
- Difficulty sleeping (insomnia)
- Itching (pruritus)
- Rash (rash)
- Itching
- Skin irritation at the application site
- Pain at the application site
- Hives (urticaria) at the application site
- Scaling at the application site (exfoliation)
- Visible blood vessels at the application site (telangiectasia)
Frequency not known (cannot be estimated from the available data):
- Blurred vision.
This medicine contains betamethasone and calcipotriol. Therefore, you may experience the following side effects. These side effects are more likely to happen if this medicine is used for a long time, if it is used under dressings or in skin folds (e.g., groin, armpits, or under the breasts), or if it is used on large areas of skin:
- allergic reactions with swelling of the face or other parts of the body, such as hands or feet. Swelling of the mouth/throat and breathing problems may also occur
- calcium levels in the blood or urine may increase so much that you have secondary symptoms. The signs are frequent urination, constipation, muscle weakness, and confusion. When treatment is stopped, calcium levels return to normal
- the adrenal glands may stop working properly. The signs are tiredness, depression, and anxiety
- blurred vision, difficulty seeing at night, sensitivity to light (this could be a sign of cataracts)
- eye pain, red eye, decreased vision, or blurred vision (could be a sign of increased pressure inside the eye)
- infections (because your immune system is weakened)
- pustular psoriasis (a red area of psoriasis with yellowish pustules [pimples])
- you may experience fluctuations in blood sugar levels.
If you experience any of the above side effects, you should contact your doctor immediately.
Among the less serious side effects that are known to be caused by calcipotriol or betamethasone are the following:
- thinning of the skin
- stretch marks
- skin blood vessels may become more visible
- changes in hair growth
- red rash around the mouth (perioral dermatitis)
- worsening of psoriasis
- skin sensitivity to light that causes a rash
- rash with itching (eczema)
- white or gray hair may temporarily change to a yellowish color at the application site when used on the scalp.
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if it is possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storing Wynzora
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton or tube after EXP. The expiry date is the last day of the month shown.
Do not store above 25°C.
Do not freeze.
Discard the tube with the remaining cream 6 months after first opening.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Contents of the pack and other information
Composition of Wynzora
- The active substances are: calcipotriol and betamethasone.
One gram of cream contains 50 micrograms of calcipotriol and 0.5 mg of betamethasone (as betamethasone dipropionate).
- The other ingredients are: isopropyl myristate, liquid paraffin, medium-chain triglycerides, isopropanol, lauryl macrogol ether, poloxamer, macrogolglycerol hydroxystearate, carbomer copolymer, butylhydroxyanisole (E320), tromethamine, disodium phosphate heptahydrate, sodium dihydrogen phosphate monohydrate, all-rac-α-tocopherol, and purified water.
Appearance of the product and pack contents
Wynzora is a white cream that comes in an aluminum tube coated with epoxifenol and with a threaded polyethylene cap.
Pack size: One 60 g tube, or a multiple pack of 120 g (2 x 60 g tubes).
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder
Almirall, S.A.
Ronda General Mitre, 151
08022 Barcelona
Spain
Manufacturer
Laboratoires Chemineau
93 route de Monnaie
Vouvray, 37210
France
This medicine is authorized in the Member States of the European Economic Area under the following names:
Denmark, Czech Republic, Finland, France, Germany, Ireland, Italy, Netherlands, Norway, Poland, Portugal, Spain, Sweden, United Kingdom (Northern Ireland): Wynzora
Austria: Winxory
Date of last revision of this leaflet: June 2025
Detailed and up-to-date information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es
- Country of registration
- Average pharmacy price44.69 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to WYNZORA 50 micrograms/g + 0.5 mg/g CreamDosage form: GEL, 50 micrograms/g 0.5 mg/gActive substance: calcipotriol, combinationsManufacturer: Aristo Pharma GmbhPrescription requiredDosage form: OINTMENT, 50 micrograms/g + 500 micrograms/gActive substance: calcipotriol, combinationsManufacturer: Teva Pharma S.L.U.Prescription requiredDosage form: OINTMENT, 50 micrograms/g + 500 micrograms/gActive substance: calcipotriol, combinationsManufacturer: Teva Pharma S.L.U.Prescription required
Online doctors for WYNZORA 50 micrograms/g + 0.5 mg/g Cream
Discuss questions about WYNZORA 50 micrograms/g + 0.5 mg/g Cream, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions








