VYVGART 1000 mg Injectable Solution

How to use VYVGART 1000 mg Injectable Solution
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Vyvgart 1000 mg Solution for Injection
efgartigimod alfa
This medicine is subject to additional monitoring, which will allow for quick identification of new safety information. You can help by reporting any side effects you may get. The last section of section 4 will tell you how to report side effects.
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Vyvgart and what is it used for
- What you need to know before you use Vyvgart
- How to use Vyvgart
- Possible side effects
- Storage of Vyvgart
- Contents of the pack and other information
1. What is Vyvgart and what is it used for
What is Vyvgart
Vyvgart contains the active substance efgartigimod alfa. Efgartigimod alfa binds to a protein in the body called neonatal Fc receptor (FcRn) and blocks it. By blocking FcRn, efgartigimod alfa reduces the level of autoantibodies against immunoglobulin G (IgG), which are immune system proteins that mistakenly attack parts of a person's body.
What Vyvgart is used for
Vyvgart is used together with reference treatment to treat adults with generalized myasthenia gravis (gMG), an autoimmune disease that causes muscle weakness. gMG can affect several muscle groups throughout the body. The disease can also cause shortness of breath, extreme fatigue, and difficulty swallowing.
In patients with gMG, autoantibodies against IgG attack and damage proteins in the nerves called acetylcholine receptors. As a result of this damage, the nerves are unable to contract the muscles normally, which causes muscle weakness and difficulty moving. By binding to the FcRn protein and reducing autoantibody levels, Vyvgart can improve muscle contraction and reduce disease symptoms and their impact on daily activities.
2. What you need to know before you use Vyvgart
Do not use Vyvgart
- if you are allergic to efgartigimod alfa or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Talk to your doctor before you start using Vyvgart.
MGFA Class V
Your doctor cannot prescribe this medicine for you if you are connected to a ventilator due to muscle weakness from gMG (myasthenic crisis).
Infections
Treatment with Vyvgart may reduce your natural resistance to infections, so you should tell your doctor if you have any infection before you start using Vyvgart.
Injection site reactions and allergic reactions
Vyvgart contains a protein that can cause reactions such as rash or itching in some people. Vyvgart can cause an anaphylactic reaction (severe allergic reaction). If you experience allergic reactions such as swelling of the face, lips, throat, or tongue that makes swallowing or breathing difficult, shortness of breath, feeling of loss of consciousness, or skin rash during or after the injection, tell your doctor immediately.
Vaccinations
Tell your doctor if you have been given any vaccine in the last 4 weeks or if you are planning to be vaccinated soon.
Children and adolescents
Do not give this medicine to children under 18 years of age, as the safety and efficacy of Vyvgart in this population have not been established.
Elderly patients
No special precautions are needed for the treatment of patients over 65 years of age.
Other medicines and Vyvgart
Tell your doctor if you are using, have recently used, or might use any other medicines.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
Driving and using machines
Vyvgart is not expected to affect your ability to drive or use machines.
Vyvgart contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per vial, which is essentially "sodium-free".
Vyvgart contains polysorbate
This medicine contains 2.7 mg of polysorbate 20 in each vial, equivalent to 0.4 mg/ml. Polysorbates can cause allergic reactions. Tell your doctor if you have any known allergy.
3. How to use Vyvgart
Follow the instructions for administration of this medicine exactly as told by your doctor or pharmacist. If you are not sure, ask your doctor or pharmacist again.
What dose of Vyvgart you will receive and how often
The recommended dose is 1000 mg administered in cycles of one injection per week for 4 weeks. Your doctor will decide when more treatment cycles are needed.
If you are already being treated with Vyvgart intravenously and want to switch to Vyvgart subcutaneously, you should receive the subcutaneous injection instead of your intravenous infusion at the start of the next treatment cycle.
Vyvgart injection
Vyvgart is given by injection under the skin (subcutaneously). You and your doctor should decide if, after proper training, you or your caregiver can inject Vyvgart. The first self-injection should be done in front of your healthcare professional. It is important that you do not try to inject Vyvgart before you have received training from a healthcare professional.
If you or your caregiver inject Vyvgart, you or your caregiver should carefully read and follow the administration instructions at the end of this leaflet (see “Important administration instructions”). Talk to your doctor, pharmacist, or nurse if you have any questions about how to administer an injection.
If you use more Vyvgart than you should
Since Vyvgart is administered in a single-use vial, it is unlikely that you will receive too much. However, if you are concerned, contact your doctor, pharmacist, or nurse for advice.
If you miss a dose or forget an appointment to receive Vyvgart
Keep a record of your next dose. It is important that you use Vyvgart exactly as your doctor has prescribed.
- If you forget to take your dose within 3 days of the date you should have taken it, take your dose as soon as you remember and then continue with your original schedule.
- If you forget to take your dose for more than 3 days, ask your doctor when you should take your next dose.
- If you miss an appointment, contact your doctor immediately for advice.
Do not take a double dose to make up for forgotten doses.
If you stop using Vyvgart
Stopping or interrupting treatment with Vyvgart may cause your gMG symptoms to come back. Talk to your doctor before stopping treatment with Vyvgart. Your doctor will explain the possible side effects and risks. Your doctor will also want to closely monitor you.
If you have any other questions about using this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Your doctor will explain the possible side effects and risks and benefits of Vyvgart before treatment.
Tell your doctor immediately if you notice:
Signs of a severe allergic reaction (anaphylactic reaction) such as swelling of the face, lips, throat, or tongue that makes swallowing or breathing difficult, shortness of breath, feeling of loss of consciousness, or skin rash during or after the injection.
If you are not sure what the following side effects are, ask your doctor to explain them to you.
Very common(may affect more than 1 in 10 people)
- infections of the nose and throat (upper respiratory tract)
- injection site reactions, which can include redness, itching, pain. These injection site reactions are usually mild to moderate and usually appear 1 day after the injection.
Common(may affect up to 1 in 10 people)
- pain or burning sensation when urinating, which can be a sign of urinary tract infection
- inflammation of the airways in the lungs (bronchitis)
- muscle pain (myalgia)
- nausea.
Frequency not known(cannot be estimated from the available data)
- allergic reactions during or after the injection.
- swelling of the face, lips, throat, or tongue that makes swallowing or breathing difficult, shortness of breath.
- pale skin, weak and rapid pulse, or feeling of loss of consciousness.
- sudden rash, itching, or hives.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Vyvgart
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and on the label after “EXP”. The expiry date refers to the last day of the month shown.
Store in a refrigerator (2°C to 8°C). Do not freeze.
If necessary, unopened vials can be stored at room temperature (up to 30°C) for a maximum of 3 days. After storage at room temperature, unopened vials can be returned to the refrigerator. The total time out of the refrigerator and at room temperature should not exceed 3 days.
Store in the original package to protect from light.
Do not use this medicine if you notice particles.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Contents and Additional Information
Vyvgart Composition
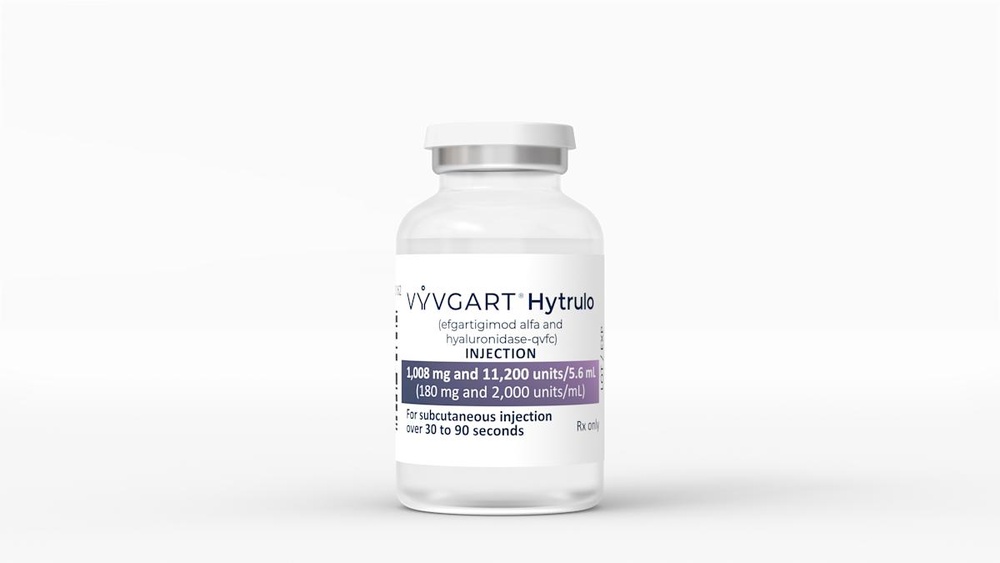
- The active ingredient is efgartigimod alfa. Each vial contains 1,000 mg of efgartigimod alfa in 5.6 ml. Each ml contains 180 mg of efgartigimod alfa.
- The other components are: recombinant human hyaluronidase (rHuPH20), L-histidine, L-histidine monohydrochloride, L-methionine, polysorbate 20, sodium chloride, sucrose, water for injectable preparations. See section 2 "Vyvgart contains sodium".
Product Appearance and Container Contents
Vyvgart is a ready-to-use, slightly yellow, transparent to slightly turbid solution, presented as a subcutaneous injectable solution.
Marketing Authorization Holder and Manufacturer
argenx BV
Industriepark-Zwijnaarde 7
9052 Gent
Belgium
You can request more information about this medicine by contacting the local representative of the marketing authorization holder:
België/Belgique/Belgien/Eesti argenx BV Tel: +32 (0) 93969394/+32 (0) 800 54477 | Lietuva argenx BV Tel: 8 800 80 052 |
| Luxembourg/Luxemburg argenx BV Tel: 800 25 233 |
Ceská republika argenx BV Tel: 800 040 854 | Magyarország argenx BV Tel.: (80) 088 578 |
Danmark argenx BV Tlf.: 80 25 41 88 | Malta argenx BV Tel: 8006 5101 |
Deutschland argenx Germany GmbH Tel: 08001803963 | Nederland argenx BV Tel: 0800 0232882 |
Ελλάδα Medison Pharma Greece Single Member Societe Anonyme Τηλ: +30 210 0100 188 | Norge argenx BV Tlf: 800 62 225 |
España argenx Spain S.L. Tel: 900 876 188 | Österreich argenx BV Tel: 0800 017936 |
France argenx France SAS Tél: +33 (0) 188898992 | Polska argenx BV Tel.: 800 005 155 |
Hrvatska argenx BV Tel: 0800 806 524 | Portugal argenx BV Tel: 800 180 844 |
Ireland/United Kingdom (Northern Ireland) argenx BV Tel: 1800 851 868 | România argenx BV Tel: 0800 360 912 |
Ísland argenx BV Sími: 800 4422 | Slovenija argenx BV Tel: 080 688955 |
Italia argenx Italia s.r.l Tel: 800729052 | Slovenská republika argenx BV Tel: 0800 002 646 |
Κύπρος argenx BV Τηλ: 80 077122 | Suomi/Finland argenx BV Puh/Tel: 0800 412838 |
Latvija argenx BV Tel: 80 205 267 | Sverige argenx BV Tel: 020‑12 74 56 |
Date of Last Revision of this Leaflet:
Other Sources of Information
Detailed information on this medicine is available on the European Medicines Agency website: https://www.ema.europa.eu. There are also links to other websites on rare diseases and orphan medicines.
--------------------------------------------------------------------------------------------------------------------Important Instructions for Use
Vyvgart 1,000 mg injectable solution
efgartigimod alfa
Subcutaneous route
Read and understand these instructions for use before administering the Vyvgart injection.
If you or your caregiver are willing to administer Vyvgart, your healthcare professional will instruct you on how to inject it. Your healthcare professional must teach you or your caregiver how to prepare and administer the Vyvgart injection correctly before using it for the first time. A demonstration of adequate self-administration under the supervision of a healthcare professional is required. It is essential that you do not attempt to inject the medicine until you have received training and you or your caregiver are sure you know how to use Vyvgart. Ask your healthcare professional if you have any questions.
Important Information to Know Before Administering a Subcutaneous Injection of Vyvgart.
- Only for subcutaneous use.
- The vial is for single use. Do notstore vials, even if they are not empty.
- Do notuse a vial if you notice unusual turbidity or visible particles. The medicine should have a slightly yellow, transparent to slightly turbid appearance.
- Do notshake the vial during handling.
- Do notuse damaged vials or those without a protective cap. Notify and return damaged vials to the pharmacy.
Storage of Vyvgart
- Store in the refrigerator (between 2 °C and 8 °C).
- Do notfreeze.
- If necessary, unopened vials can be stored at room temperature (up to 30 °C) for a maximum of 3 days. After storage at room temperature, unopened vials can be returned to the refrigerator. The total time outside the refrigerator and at room temperature should not exceed 3 days.
- Store in the original packaging to protect it from light.
- Keep this medicine out of the sight and reach of children.
| |
1 vial containing Vyvgart |
|
Vyvgart leaflet and instructions for use |
|
Additional Material Not Included Store additional material at room temperature in a dry place | |
Alcohol wipes |
|
10 ml syringe |
|
18-gauge, ≥ 38 mm long transfer needle |
|
25-gauge, 30 cm long microinfusion set, maximum priming volume of 0.4 ml |
|
Sterile gauze |
|
Adhesive dressing |
|
Sharps container |
|
Material Preparation
Step1 Remove the vial box from the refrigerator. |
|
Step2 Remove the vial from the box and check that:
If any of the above conditions are not met, do notadminister the injection and inform the pharmacy. |
|
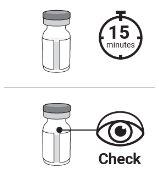
Step3 Wait at least 15 minutes for the vial to reach room temperature naturally. Check if the medicine in the vial is slightly yellow, transparent to slightly turbid, and does not contain visible particles.
|
|
Step4 Gather the following additional material:
| |
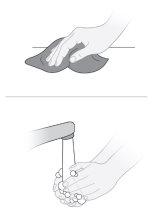
Step5 5a.Clean the work area. 5b.Wash your hands with soap and dry them well. |
|
Syringe Preparation | |
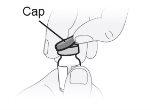
Step6 Remove the removable plastic protective cap from the vial. The aluminum seal should remain in place. |
|
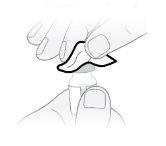
Step7 Clean the rubber stopper with a new alcohol wipe. Let it air dry naturally for at least 30 seconds. Do notblow on the rubber stopper. |
|
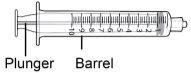
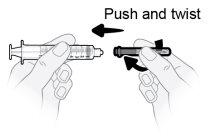
Step8 Unwrap the syringe and transfer needle. Insert the transfer needle into the syringe and twist it clockwise until the needle is firmly attached to the syringe. Do nottouch the tip of the syringe or the lower part of the needle to avoid germs and infection risk. |
|
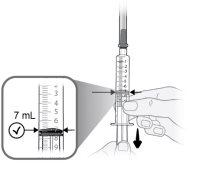
Step9 Slowly pull the plunger and draw up to 7 ml of air into the syringe. |
|
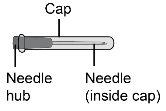
Step10 10a.Hold the syringe by the cone where the needle is attached to the syringe. 10b.Hold the transfer needle cap and carefully pull it off the body. 10c.Place the transfer needle cap down on a clean, flat surface.
|
|
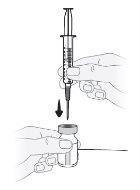
Step11 Hold the vial upright on a flat surface and insert the transfer needle through the center of the disinfected rubber stopper. Do notpuncture the rubber stopper of the vial more than once to avoid leaks. |
|
Step12 Turn the vial upside down while keeping the transfer needle inserted in it. |
|
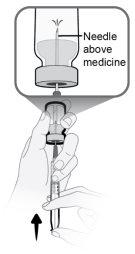
Step13 13a.Make sure the transfer needle in the vial points upwards with the needle tip above the medicine solution. 13b.Gently push the plunger to inject all the air from the syringe into the empty space above the medicine solution in the vial. 13c.Keep your finger pressed on the syringe plunger. Do notinject air into the medicine solution, as air bubbles or foam may be created. |
|
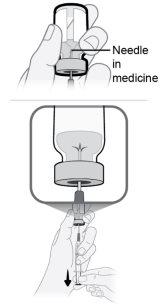
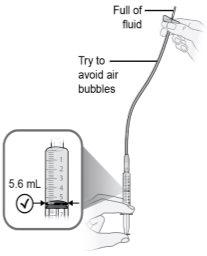
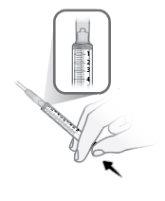
Step14 Fill the syringe as follows: 14a.Keep your finger pressed on the syringe plunger and slide the tip of the transfer needle into the medicine solution in the neck of the vial (near the vial cap) so that the tip of the needle is completely covered in the solution. 14b.Slowly pull the plunger back, keeping the tip of the transfer needle in the solution to avoid air bubbles and foam in the syringe. Fill the syringe with the entire contents of the vial. |
|
Step15 Remove large air bubbles, if any. 15a.Keep the transfer needle in the vial and check if there are large air bubbles in the syringe. 15b.Remove large air bubbles by gently tapping the syringe with your fingers until the bubbles rise to the top of the syringe. 15c.Move the tip of the transfer needle above the medicine solution and slowly push the plunger up to expel the air bubbles from the syringe. 15d.To remove any remaining medicine solution from the vial, reinsert the tip of the transfer needle into the solution and slowly pull the plunger back until you have the entire contents of the vial in the syringe. 15e.Repeat the above steps until the large air bubbles are removed. If you cannot withdraw all the liquid from the vial, place it in an upright position to reach the remaining amount. |
|
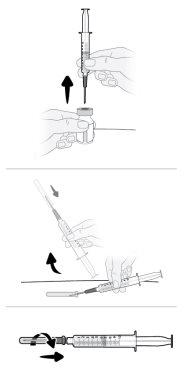
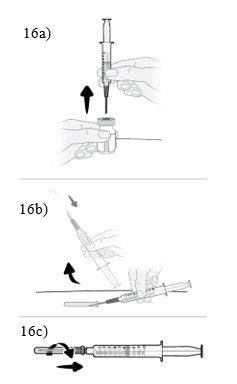
| Step16 16a.Turn the vial into a vertical position and remove the syringe and transfer needle from the vial. 16b.With one hand, slide the transfer needle into the cap and lift to cover the needle. 16c.Once the transfer needle is covered, screw the cap onto the syringe to secure it completely. |
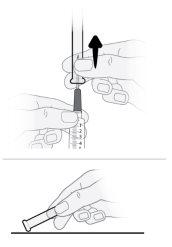
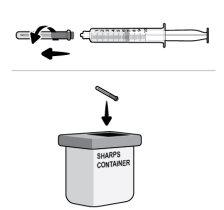
Step17 17a.Gently pull the transfer needle and turn it counterclockwise to remove it from the syringe. 17b.Discard (throw away) the transfer needle in the sharps disposal container. |
|
Preparing the Vyvgart Injection
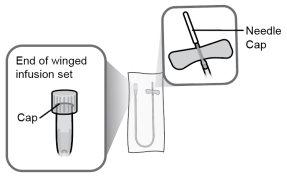
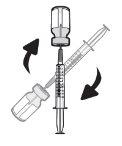
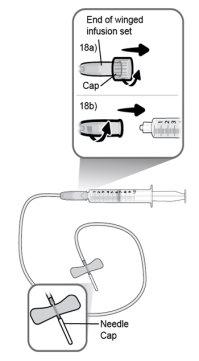
Step18 18a.Remove the cap from the end of the microinfuser. 18b.Gently push and turn the end of the microinfuser clockwise onto the syringe until it is firmly attached. The final configuration of the syringe should resemble the figure on the right.
|
|
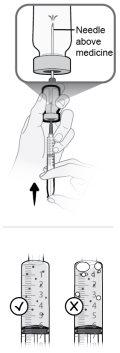
Step19 19a.Fill the microinfuser tube by gently pressing the syringe plunger until it reaches the 5.6 ml mark. A small amount of liquid should be visible at the tip of the needle. 19b.Place the syringe and attached microinfuser on a clean and flat surface. Do notclean the excess medication solution expelled from the infusion equipment during the filling of the tube. |
|
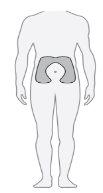
Step20 Choose the injection site
Choose a different injection site each time you inject (rotate the site) to reduce discomfort. Note: Do notinject into areas where the skin is red, bruised, sensitive, hard, or has moles or scars. |
|
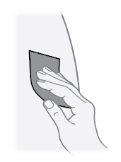
Step21 Disinfect the injection site with a new alcohol swab. Make circular movements and clean from the inside out. Let the area air dry for at least 30 seconds. Do nottouch the injection site after disinfecting it. |
|
Vyvgart Injection
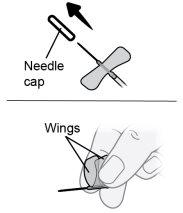
Step22 22a.Carefully remove the needle cap from the microinfuser. 22b.Fold the wings of the infusion set upwards and hold them between your thumb and index finger, with the needle below the wings. Note: To avoid infections, make sure the needle does not come into contact with anything before skin insertion. |
|
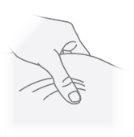
Step23 With your free hand, pinch a fold of skin around the disinfected injection site and lift it upwards. Take enough skin to create a "tent" to insert the needle into. Do nothold the skin too tightly to avoid bruising. |
|
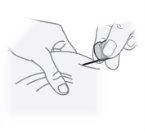
Step24 Insert the needle into the center of the pinched skin area at an angle of about 45 degrees. Note: The needle should be inserted smoothly into the skin. If you feel resistance, you can gently pull the needle back. |
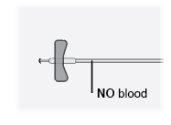
|
Step25 Check the infusion equipment. Make sure there is no blood. Important: If you see blood, gently pull the needle back without removing it from the skin. |
|
Step26 Administer the injection by pushing the syringe plunger with constant pressure until no medication remains in the syringe. This corresponds to the injection of the recommended dose of 5.6 ml. The injection usually takes between 30 and 90 seconds. Note:
|
|
Step27 27a.Once all the solution has been injected, remove the needle from the skin. 27b.Cover the injection site with a sterile dressing, such as an adhesive bandage. Note: If a small drop of blood is observed after removing the needle, do notworry. This can occur if the needle punctures the skin during extraction. Clean the blood with a sterile gauze and press gently. No further bleeding should occur. Apply a sterile dressing to cover the area. |
|
Disposal of Vyvgart
Step28 Discard (throw away) the microinfuser (with the needle and syringe attached) and the vial in the sharps disposal container. If you do nothave a sharps disposal container, you can use a household container if it:
Discard the full container according to the instructions of your doctor or pharmacist. Note: Always keep the sharps disposal container out of sight and reach of children. |
|
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to VYVGART 1000 mg Injectable SolutionDosage form: INJECTABLE, 1000 mgActive substance: efgartigimod alfaManufacturer: Argenx B.V.Prescription requiredDosage form: INJECTABLE PERFUSION, 20 mg/mlActive substance: efgartigimod alfaManufacturer: Argenx B.V.Prescription requiredDosage form: TABLET, 180 mgActive substance: mycophenolic acidManufacturer: Laboratorio Stada S.L.Prescription required
Online doctors for VYVGART 1000 mg Injectable Solution
Discuss questions about VYVGART 1000 mg Injectable Solution, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions






 Container Contents
Container Contents