VOCABRIA 600 mg PROLONGED-RELEASE INJECTABLE SUSPENSION

How to use VOCABRIA 600 mg PROLONGED-RELEASE INJECTABLE SUSPENSION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Vocabria 600 mg prolonged-release injectable suspension
cabotegravir
This medicinal product is subject to additional monitoring, which will allow for quick identification of new safety information. You can help by reporting any side effects you may get. The last section of the package leaflet contains information on how to report side effects.
Read all of this leaflet carefully before you start using this medicine, because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or nurse.
- If you get any side effects, talk to your doctor or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Vocabria and what is it used for
- What you need to know before you are given Vocabria
- How Vocabria is given
- Possible side effects
- Storing Vocabria
- Package contents and further information
1. What is Vocabria and what is it used for
Vocabria injectable contains cabotegravir as the active substance. Cabotegravir belongs to a group of antiretroviral medicines called integrase inhibitors (INI).
Vocabria injectable is used to treat HIV (Human Immunodeficiency Virus) infection in adults and adolescents (at least 12 years of age and weighing at least 35 kg), who are also taking another antiretroviral injectable medicine called rilpivirine and whose HIV-1 infection is under control.
Vocabria injections do not cure HIV infection; they keep the amount of virus in your body at a low level. This helps to keep the number of CD4+ cells in your blood at a normal level. CD4+ cells are a type of white blood cell that are important for helping your body to fight infections.
Vocabria injections are always givenin combination with another injection of another antiretroviral medicine called rilpivirine injectable. Refer to the package leaflet for rilpivirine for more information about this medicine.
2. What you need to know before you are given Vocabria
Do not receive Vocabria injectable
- if you have ever had a severe skin rash, skin peeling, blisters, and/or mouth sores.
- if you are allergic(hypersensitive) to cabotegravir or any of the other ingredients of this medicine (listed in section 6).
- if you are taking any of these medicines as they may affect how Vocabria works:
- carbamazepine, oxcarbazepine, phenytoin, phenobarbital(medicines to treat epilepsy and prevent seizures)
- rifampicinor rifapentine(medicines to treat some bacterial infections, such as tuberculosis).
If you think this applies to you, tell your doctor.
Warnings and precautions
Severe skin reaction
Very rare severe skin reactions, Stevens-Johnson syndrome, and toxic epidermal necrolysis have been reported in association with Vocabria. If you notice any of the symptoms related to these severe skin reactions, do not receive the next Vocabria injection and seek medical attention immediately.
Read the informationin section 4 of this leaflet (“Possible side effects”).
Allergic reaction
Vocabria contains cabotegravir, which is an integrase inhibitor. Integrase inhibitors, including cabotegravir, can cause a severe allergic reaction known as hypersensitivity reaction. You need to know what the important signs and symptoms are to look out for while you are taking Vocabria.
Read the informationin section 4 of this leaflet.
Liver problems including hepatitis B and/or C
Tell your doctor if you have or have had liver problems, including hepatitis B and/or C. Your doctor will assess the severity of your liver disease before deciding if you can take Vocabria.
Be aware of important symptoms
Some people who take medicines for HIV infection develop other conditions, which can be serious.
You need to know the important signs and symptoms to look out for while you are being treated with Vocabria. These include:
- symptoms of infection
- symptoms of liver damage
Read the informationin section 4 of this leaflet (“Possible side effects”).
If you have symptoms of infection or liver damage:
Tell your doctor immediately. Do not take other medicines for infection unless your doctor advises you to.
Regular visits are important
It is important that you attend your scheduled appointmentsto receive your Vocabria injection, to monitor your HIV infection, and to prevent your disease from getting worse. Tell your doctor if you are thinking of stopping treatment. If you miss a Vocabria injection, or if you stop receiving Vocabria, you will need to take other medicines to treat your HIV infection and to reduce the risk of developing viral resistance.
Vocabria injectable is a long-acting medicine.If you stop treatment, low levels of cabotegravir (the active substance of Vocabria) may remain in your body for up to 12 months or more after the last injection. These low levels of cabotegravir will not protect you from the virus, and the virus may become resistant. You should start a different HIV treatment within one month after the last Vocabria injection if you are receiving monthly injections, and within two months after the last Vocabria injection if you are receiving injections every two months.
Children and adolescents
This medicine must not be used in children under 12 years of age or adolescents weighing less than 35 kg, as it has not been studied in these patients.
Other medicines and Vocabria injectable
Tell your doctor if you are taking, have recently taken, or might take any other medicines, including medicines obtained without a prescription.
Vocabria must not be administeredwith other medicines (see ‘Do not receive Vocabria injectable’ above in section 2).
Some medicines may affect how Vocabria worksor increase the chance of you having side effects. Vocabria may also affect how other medicines work.
Tell your doctorif you are taking:
- rifabutin(to treat some bacterial infections, such as tuberculosis).
Tell your doctoror pharmacist if you are taking this medicine. Your doctor may decide that you need extra checks.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby:
Ask your doctorfor advice before receiving Vocabria injectable.
Pregnancy
- Vocabria is not recommended during pregnancy.If necessary, your doctor will consider the benefit to you and the risk to your baby of receiving Vocabria injections while you are pregnant. If you are planning to become pregnant, talk to your doctor beforehand.
- If you become pregnant, do not stop attending your appointments to receive your Vocabria injection without consulting your doctor.
Breastfeeding
It is not recommendedthat HIV-positive women breastfeed their babies because HIV infection can be passed to the baby through breast milk.
It is not knownwhether the components of Vocabria injectable can pass into breast milk. However, it is possible that cabotegravir could pass into breast milk during the 12 months following the last Vocabria injection.
If you are breastfeeding or thinking of breastfeeding, consult your doctor as soon as possible.
Driving and using machines
Vocabria may make you feel dizzyand have other side effects that make you less alert.
Do not drive or use machinesunless you are sure that you are not affected.
Important information about some of the ingredients of Vocabria
The Vocabria injection contains polysorbate. This medicine contains 60 mg of polysorbate per 3 ml dose. Tell your doctor if you have any known allergy.
3. How Vocabria is given
You will be given Vocabria by injection, once a month or once every 2 months, together with another injectable medicine called rilpivirine. Your doctor will tell you about the dosing schedule.
A nurse or doctor will give you Vocabria by injection into the muscle of your buttock (intramuscular, or IM, injection).
When you start treatmentwith Vocabria, you and your doctor may decide to start treatment with Vocabria tablets or go straight to Vocabria injections. If you decide to start with the tablets, your doctor will tell you:
- to take one Vocabria 30 mg tablet and one rilpivirine 25 mg tablet, once a day, for about one month
- after that, you will receive the injectionseither monthly or every 2 months.
This first month of Vocabria and rilpivirine tablets is called the oral lead-in period. It will allow your doctor to check if it is suitable for you to switch to the injections.
Monthly injection schedule
Which medicine | When | |
First injection | Second injection onwards, every month | |
Vocabria | 600 mg injection | 400 mg injection every month |
Rilpivirine | 900 mg injection | 600 mg injection every month |
Every 2 months injection schedule
Which medicine | When | |
First and second injection, one month apart | Third injection onwards, every 2 months | |
Vocabria | 600 mg injection | 600 mg injection every 2 months |
Rilpivirine | 900 mg injection | 900 mg injection every 2 months |
If you miss a Vocabria injection
?Contact your doctor immediatelyto schedule a new appointment
It is important to control your HIV and prevent your disease from getting worse, that you go to your scheduled appointments to receive your Vocabria injection. Talk to your doctor if you are thinking of stopping treatment.
Talk to your doctorif you think you will not be able to receive your Vocabria injection as scheduled. Your doctor may recommend that you take Vocabria tablets or another HIV treatment instead, until you can receive your Vocabria injection again.
If you are given more Vocabria injectable than you should
This medicine will be given to you by a doctor or nurse, so it is unlikely that you will be given too much. If you are concerned, talk to your doctor or nurse.
Do not stop receiving Vocabria injections without your doctor’s advice.
Keep receiving Vocabria injections for as long as your doctor recommends. Do not stop unless your doctor tells you to. If you stop treatment, your doctor should start you on another HIV treatment within one month after the last Vocabria injection if you are receiving monthly injections, and within two months after the last Vocabria injection if you are receiving injections every two months, to reduce the risk of developing viral resistance.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible Adverse Effects
Like all medicines, this medicine can cause adverse effects, although not all people suffer from them.
Do not receive the next Vocabria injection and seek immediate medical attentionif you notice any of the following symptoms:
- red, non-raised, target-like or circular patches on the trunk, often with blisters in the center, skin peeling, sores in the mouth, throat, nose, genitals, and eyes. These severe skin eruptions can be preceded by fever and flu-like symptoms (Stevens-Johnson syndrome, toxic epidermal necrolysis). These severe skin reactions are very rare (may affect up to 1 in 10,000people).
Allergic Reactions
Vocabria contains cabotegravir, which is an integrase inhibitor. Integrase inhibitors, including cabotegravir, can cause a severe allergic reaction known as a hypersensitivity reaction. These hypersensitivity reactions are uncommon (may affect up to 1 in 100people).
If you experience any of the following symptoms:
- skin reaction (rash, hives)
- high temperature (fever)
- lack of energy (fatigue)
- swelling, sometimes of the face or mouth (angioedema), which causes difficulty breathing
- muscle or joint pain.
See a doctor immediately.Your doctor may consider it necessary to perform tests to check your liver, kidneys, or blood and may advise you to stop taking Vocabria.
Very Common Adverse Effects
These may affect more than 1 in 10people:
- headache
- reactions at the injection site. In clinical studies, most were generally mild to moderate and became less frequent over time. Symptoms may include:
- pain (which can rarely include temporary difficulty walking) and discomfort, a lump or hardened mass
- feeling of heat (pyrexia), which can occur in the first week after injections.
Common Adverse Effects
These may affect up to 1 in 10people:
- depression
- anxiety
- abnormal dreams
- difficulty sleeping (insomnia)
- dizziness
- nausea
- vomiting
- stomach pain (abdominal pain)
- gas (flatulence)
- diarrhea
- rash
- muscle pain (myalgia)
- lack of energy (fatigue)
- feeling of weakness (asthenia)
- general malaise
- weight gain.
- reactions at the injection site. In clinical studies, most were generally mild to moderate and became less frequent over time. Symptoms may include: redness, itching, swelling, heat, bruising (which can include discoloration or a collection of blood under the skin).
Uncommon Adverse Effects
These may affect up to 1 in 100people:
- suicide attempts and suicidal thoughts (especially in patients who have previously had depression or mental health problems)
- allergic reaction (hypersensitivity)
- hives (urticaria)
- swelling, sometimes of the face or mouth (angioedema), which causes difficulty breathing
- feeling of drowsiness
- feeling of dizziness, during or after an injection. This can lead to fainting
- liver damage (signs may include yellowing of the skin and whites of the eyes, loss of appetite, itching, stomach tenderness, pale stools, or abnormally dark urine)
- changes in liver markers in blood tests (increased transaminasesor increased bilirubin).
- reactions at the injection site. In clinical studies, most were generally mild to moderate and became less frequent over time. Symptoms may include: numbness, mild bleeding, an abscess (collection of pus), or cellulitis (heat, swelling, or redness).
Other Adverse Effects that May Appear in Blood Tests
- an increase in lipase (a substance produced by the pancreas).
Other Possible Adverse Effects
People receiving HIV treatment with Vocabria and rilpivirine may have other adverse effects.
Pancreatitis
If you have severe abdominal pain (stomach), this may be due to pancreas inflammation (pancreatitis).
Tell your doctor,especially if the pain spreads and worsens.
Symptoms of Infection and Inflammation
People with advanced HIV infection (AIDS) have a weakened immune system and are more prone to developing severe infections (opportunistic infections). When they start treatment, the immune system strengthens, so the body begins to fight these infections.
Symptoms of infection and inflammation can develop, caused by:
- latent old infections that reappear as the body fights them
- the immune system attacking healthy tissues (autoimmune disorders).
Symptoms of autoimmune disorders may appear many months after starting HIV treatment.
Symptoms may include:
- muscle weaknessand/or muscle pain
- painor swelling of the joints
- weaknessthat starts in the hands and feet and moves up to the body trunk
- palpitationsor tremors
- hyperactivity(excessive restlessness and movement).
If you have any symptoms of infection:
Tell your doctor immediately.Do not take other medicines for the infection without consulting your doctor first.
Reporting Adverse Effects
If you experience any type of adverse effect, consult your doctor or nurse, even if it is a possible adverse effect that is not listed in this leaflet. You can also report them directly through the national reporting system included in Appendix V. By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Storage of Vocabria
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiration date stated on the label and carton after CAD. The expiration date is the last day of the month indicated.
Do not freeze.
6. Container Contents and Additional Information
Vocabria Composition
- The active ingredient is cabotegravir.
Each 3 ml vial contains 600 mg of cabotegravir.
The other ingredients are:
Manitol (E421)
Polysorbate 20 (E432)
Macrogol (E1521)
Water for injectable preparations
Product Appearance and Container Contents
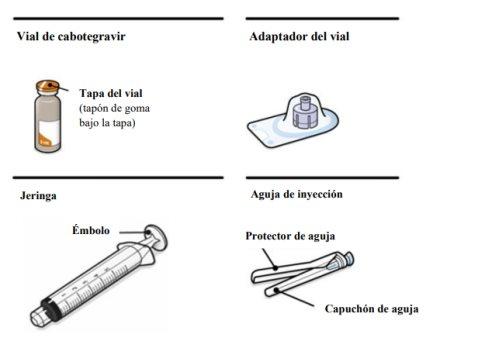
Cabotegravir prolonged-release injectable suspension is presented in a topaz-colored glass vial with a rubber stopper. The container also includes 1 syringe, 1 vial adapter, and 1 injection needle.
Marketing Authorization Holder
ViiV Healthcare BV
Van Asch van Wijckstraat 55H
3811 LP Amersfoort
Netherlands
Manufacturer
GlaxoSmithKline Manufacturing SpA
Strada Provinciale Asolana, 90
San Polo di Torrile
Parma, 43056
Italy
You can request more information about this medication by contacting the local representative of the marketing authorization holder:
Belgium/Belgique/Belgien ViiV Healthcare srl/bv Tel: + 32 (0) 10 85 65 00 | Lithuania ViiV Healthcare BV Tel: + 370 80000334 |
ViiV Healthcare BV Tel: + 359 80018205 | Luxembourg/Luxemburg ViiV Healthcare srl/bv Belgique/Belgien Tel: + 32 (0) 10 85 65 00 |
Czech Republic GlaxoSmithKline, s.r.o. Tel: + 420 222 001 111 | Hungary ViiV Healthcare BV Tel: + 36 80088309 |
Denmark GlaxoSmithKline Pharma A/S Tel: + 45 36 35 91 00 | Malta ViiV Healthcare BV Tel: + 356 80065004 |
Germany ViiV Healthcare GmbH Tel: + 49 (0)89 203 0038-10 | Netherlands ViiV Healthcare BV Tel: + 31 (0) 33 2081199 |
Estonia ViiV Healthcare BV Tel: + 372 8002640 | Norway GlaxoSmithKline AS Tel: + 47 22 70 20 00 |
Greece GlaxoSmithKline Μονοπρóσωπη A.E.B.E. Tel: + 30 210 68 82 100 | Austria GlaxoSmithKline Pharma GmbH Tel: + 43 (0)1 97075 0 |
Spain Laboratorios ViiV Healthcare, S.L. Tel: + 34 900 923 501 | Poland GSK Services Sp. z o.o. Tel: + 48 (0)22 576 9000 |
France ViiV Healthcare SAS Tel: + 33 (0)1 39 17 69 69 | Portugal VIIVHIV HEALTHCARE, UNIPESSOAL, LDA Tel: + 351 21 094 08 01 |
Croatia ViiV Healthcare BV Tel: + 385 800787089 | Romania ViiV Healthcare BV Tel: + 40 800672524 |
Ireland GlaxoSmithKline (Ireland) Limited Tel: + 353 (0)1 4955000 | Slovenia ViiV Healthcare BV Tel: + 386 80688869 |
Iceland Vistor ehf. Tel: +354 535 7000 | Slovakia ViiV Healthcare BV Tel: + 421 800500589 |
Italy ViiV Healthcare S.r.l Tel: + 39 (0)45 7741600 | Finland GlaxoSmithKline Oy Tel: + 358 (0)10 30 30 30 |
Cyprus ViiV Healthcare BV Tel: + 357 80070017 | Sweden GlaxoSmithKline AB Tel: + 46 (0)8 638 93 00 |
Latvia ViiV Healthcare BV Tel: + 371 80205045 |
Date of Last Revision of this Prospectus:<{MM/AAAA}.
Other Sources of Information
Detailed information about this medication is available on the European Medicines Agency website: https://www.ema.europa.eu
------------------------------------------------------------------------------------------------------------------
This information is intended solely for healthcare professionals:
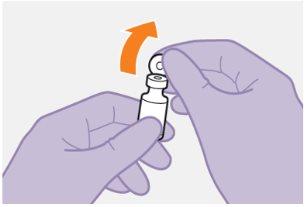
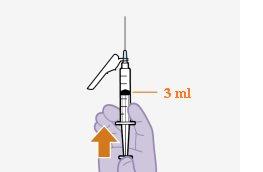
Instructions for Use of Vocabria 3 ml Injectable:
General Description A complete dose requires two injections: VOCABRIA and rilpivirina 3 ml of cabotegravir and 3 ml of rilpivirina. Cabotegravir and rilpivirina are suspensions that do not require further dilution or reconstitution. The preparation steps for both medications are the same. Follow these instructions carefully when preparing the injectable suspension to avoid leakage. Cabotegravir and rilpivirina are for intramuscular use only. Both injections should be administered in the buttocks. Note:The ventrogluteal area is recommended. The order of administration is not important. | ||
Storage Information | ||
| ||
Your Container Contains | ||
Consider the patient's constitution and medical judgment to choose the appropriate needle length.
| ||
You Will Also Need | ||
Make sure you have the rilpivirina container before starting.
| ||
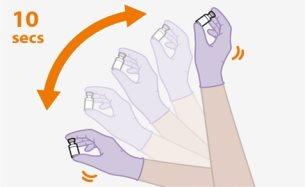
Preparation | ||
| ||
|
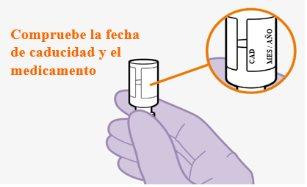
Note:The cabotegravir vial is topaz-colored. Do not useif the expiration date has passed. | |
| ||
|
| |
| ||
|
| |
| ||
|
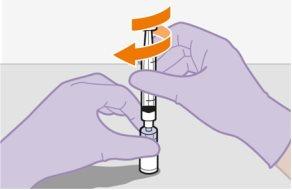
Note:The order of preparation of the vials is not important. | |
| ||
|
| |
| ||
|
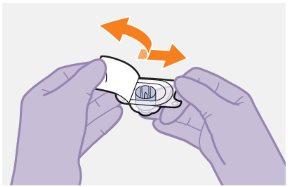
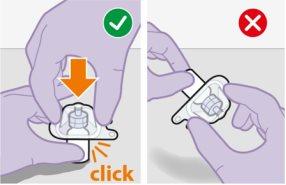
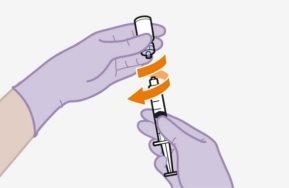
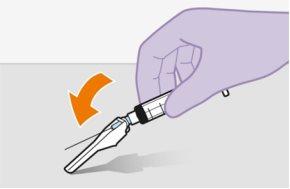
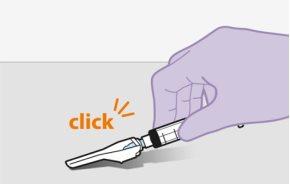
Note:Do notremove the adapter from its packaging until the next step. The adapter will notfall out when turning the packaging over. | |
| ||
|
| |
| ||
|
| |
| ||
|
| |
| ||
|
| |
| ||
|
| |
| ||
|
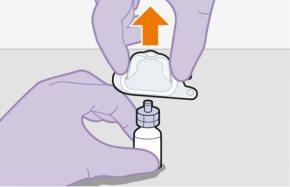
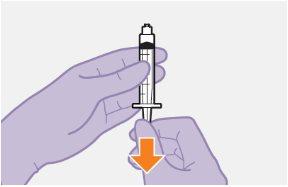
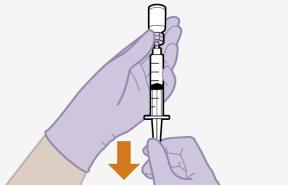
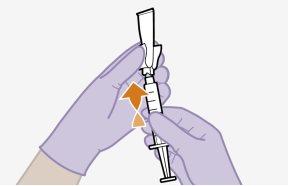
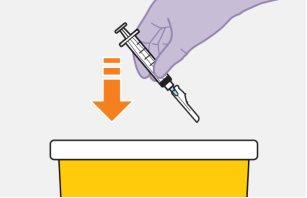
Note:Keep the syringe facing up to avoid dripping. | |
| ||
|
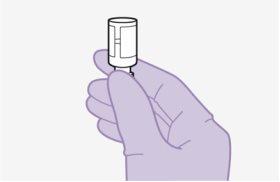
Note:Check that the cabotegravir suspension appears uniform and is white to light pink in color. | |
| ||
|
| |
Injection | ||
| ||
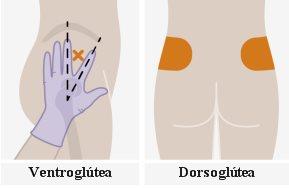
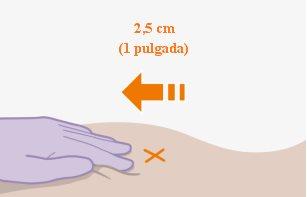
| The injections should be administered in the buttocks. Choose between the following areas for injection:
Note:For intramuscular use in the buttock only. Do notinject intravenously. | |
| ||
|
| |
| ||
|
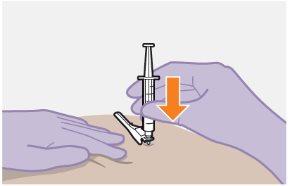
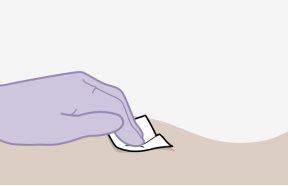
Note:Clean the injection site with an alcohol-impregnated swab. Allow the skin to air dry before proceeding. | |
| ||
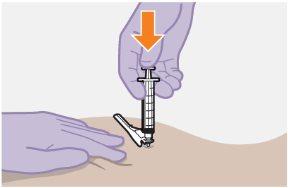
| Use the Z-track injection technique to minimize medication leakage at the injection site.
| |
| ||
|
| |
| ||
|
| |
| ||
|
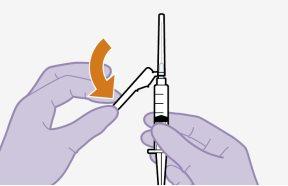
Do notmassage the area. | |
| ||
|
| |
After Injection | ||
| ||
|
| |
Repeat for the Second Medication | ||
| If you have not yet injected both medications, follow the preparation and injection steps for rilpivirina, which has its own instructions for use. | |
Questions and Answers | ||
The injection should be used immediately after the suspension has been loaded into the syringe, from a microbiological point of view. Chemical and physical stability has been demonstrated for 2 hours at 25°C.
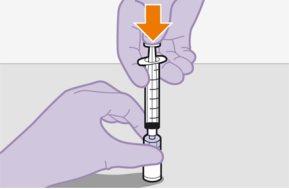
Injecting 1 ml of air into the vial facilitates the withdrawal of the dose with the syringe. Without the air, some of the liquid may return to the vial unintentionally, leaving less than expected in the syringe.
No, the order is not important.
It is best to let the vial reach room temperature naturally. However, you can use the heat from your hands to speed up the temperature adjustment time. Do not use any other heating method. 5. Why is administration in the ventrogluteal area recommended? Administration in the ventrogluteal area is recommended, in the middle gluteal muscle, because it is farther from the main nerves and blood vessels. Administration in the dorsogluteal area, in the gluteus maximus muscle, is also acceptable if preferred by the healthcare professional. The injection should not be administered in any other area. |
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to VOCABRIA 600 mg PROLONGED-RELEASE INJECTABLE SUSPENSIONDosage form: TABLET, 30 mgActive substance: cabotegravirManufacturer: Viiv Healthcare B.V.Prescription requiredDosage form: INJECTABLE, 600 mgActive substance: cabotegravirManufacturer: Viiv Healthcare B.V.Prescription requiredDosage form: TABLET, 30 mgActive substance: cabotegravirManufacturer: Viiv Healthcare B.V.Prescription required
Online doctors for VOCABRIA 600 mg PROLONGED-RELEASE INJECTABLE SUSPENSION
Discuss questions about VOCABRIA 600 mg PROLONGED-RELEASE INJECTABLE SUSPENSION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions