
VIZIBIM 0.3 mg/ml EYE DROPS SOLUTION

How to use VIZIBIM 0.3 mg/ml EYE DROPS SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
VIZIBIM 0.3 mg/ml, eye drops solution
bimatoprost
Read the package leaflet carefully before you start using the medicine, because it contains important information for you.
- Keep this package leaflet, you may need to read it again. If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this package leaflet. See section 4.
Contents of the package leaflet
- What is VIZIBIM and what is it used for
- What you need to know before you use VIZIBIM
- How to use VIZIBIM
- Possible side effects
- Storage of VIZIBIM
- Contents of the pack and other information
1. What is VIZIBIM and what is it used for
VIZIBIM is a medicine for glaucoma. It belongs to a group of medicines called prostamides.
VIZIBIM eye drops are used to reduce high pressure in the eye. This medicine can be used alone or with other eye drops called beta blockers, which also reduce pressure.
The eye contains a clear watery liquid that nourishes the inside of the eye. This liquid is constantly drained out of the eye and at the same time new liquid is formed to replace it. If the liquid does not drain quickly enough, the pressure inside the eye increases. This medicine works by increasing the drainage of the liquid. This reduces the pressure inside the eye. If the high pressure inside the eye is not reduced, it could lead to a disease called glaucoma and damage your vision.
VIZIBIM eye drops solution is a sterile solution that does not contain preservatives.
2. What you need to know before you use VIZIBIM
Do not use VIZIBIM:
- If you are allergic to bimatoprost or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Talk to your doctor or pharmacist before you start using VIZIBIM.
Talk to your doctor if:
- you have any respiratory problems;
- you have kidney or liver problems;
- you have had cataract surgery in the past;
- you have dry eye;
- you have or have had any problems with the cornea (the transparent front part of the eye);
- you have or have had low blood pressure or heart rate;
- you have had a viral infection or eye inflammation.
During treatment, VIZIBIM may cause fat loss around the eyes that can cause deepening of the palpebral sulcus, sinking of the eyes (enophthalmos), drooping of the eyelids (ptosis), stretching of the skin around the eye (involution of the dermatocalasis), and the white part of the eye becomes more visible (inferior scleral exposure). The changes are usually mild, but if they worsen, they can affect your field of vision. The changes may disappear if you stop using VIZIBIM. VIZIBIM may also cause darkening and growth of your eyelashes, as well as darkening of the skin around the eyelid. The color of the iris may darken. These changes may be permanent. The changes could be more noticeable if only one eye is treated.
Children and adolescents
This medicine has not been studied in patients under 18 years of age, and therefore VIZIBIM should not be used in patients under 18 years of age.
Other medicines and VIZIBIM
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
If you use VIZIBIM with another eye medicine, wait at least five minutes between using VIZIBIM and the other medicine.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
You should not use VIZIBIM during breastfeeding, as this medicine may pass into breast milk.
Driving and using machines
After using this medicine, you may notice that your vision becomes blurry for a short time. In this case, do not drive or use machines until your vision is clear again.
VIZIBIM contains disodium phosphate heptahydrate
This medicine contains 0.042 mg of phosphates in each drop, which is equivalent to 1.056 mg/ml.
If you have severe damage to the transparent layer on the front of the eye (the cornea), phosphates may cause, in very rare cases, cloudy spots on the cornea due to calcium accumulation during treatment.
3. How to use VIZIBIM
Follow the instructions for administration of this medicine exactly as indicated by your doctor or pharmacist. If you are unsure, talk to your doctor or pharmacist again.
This medicine should only be applied to the eye. The recommended dose is one drop of VIZIBIM once a day, at night, in each eye that needs treatment.
If you use VIZIBIM with another eye medicine, wait at least five minutes between using VIZIBIM and the other medicine.
Do not use this medicine more than once a day, as it may reduce the effectiveness of the treatment.
Avoid touching the dropper tip to the eye or the surrounding areas. This could damage the eye. The solution could become contaminated with bacteria that could cause eye infections and lead to serious damage to the eye, including vision loss.
To avoid possible contamination of the multidose container, avoid touching the dropper tip to any surface.
Instructions for use
Before applying the eye drops:
- Wash your hands before opening the bottle.
- Do not use this medicine if you notice that the security seal of the bottle is broken before using it for the first time.
- When you use it for the first time, before applying a drop to the eye, you must first test the dropper by slowly squeezing it until a drop falls into the air, away from the eye.
- When you feel confident to apply a drop in a controlled manner, decide which position you are most comfortable in to apply the drops (you can sit, lie down, or stand in front of a mirror).
Instillation:
- Hold the bottle directly under the cap and turn the cap to open the bottle. The tip of the bottle should not come into contact with anything to avoid contaminating the solution.
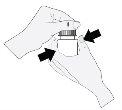
- Tilt your head back and hold the bottle above the eye.
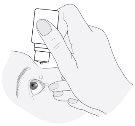
- Pull the lower eyelid down and look up. Gently squeeze the bottle in the middle and let one drop fall into the eye. Note that it may take a few seconds from when you squeeze the bottle until the drop comes out. Do not squeeze too hard.
If you are unsure about how to use this medicine, talk to your doctor or pharmacist.

- Blink a few times to spread the drop throughout the eye.
- Repeat the instructions from steps 2 to 4 to apply a drop to the other eye, if your doctor has instructed you to do so. Sometimes only one eye needs to be treated. Your doctor will tell you if this is the case and which eye needs treatment.
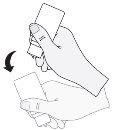
- After application, and before closing the bottle with the cap, shake the bottle once downwards, without touching the dropper tip, to remove any residual liquid from the tip. This is necessary to allow for the subsequent application of more drops.
- After you have applied all the doses, some VIZIBIM will remain in the bottle. Do not worry, as an additional amount of VIZIBIM is included, and you will receive the full amount of VIZIBIM prescribed by your doctor. Once the treatment cycle is completed, do not attempt to use the excess medicine remaining in the bottle.
Do not use the eye drops for more than 28 days after first opening the bottle.
If you use more VIZIBIM than you should
If you use more VIZIBIM than you should, it is unlikely to cause you any serious harm. Apply the next dose at the usual time. If you have any doubts, talk to your doctor or pharmacist.
If you forget to use VIZIBIM
If you forget to apply VIZIBIM, apply one drop as soon as you remember, and then return to your usual routine. Do not apply a double dose to make up for the forgotten dose.
If you stop using VIZIBIM
VIZIBIM should be used daily for it to work properly. If you stop using VIZIBIM, the pressure in your eye may increase, so talk to your doctor before stopping this treatment.
If you have any further questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Very common:may affect more than 1 in 10 people
Affecting the eye:
- Lengthening of the eyelashes (up to 45% of people)
- Mild redness (up to 44% of people)
- Itching (up to 14% of people)
- Fat loss in the eye area that can cause deepening of the palpebral sulcus, sinking of the eyes (enophthalmos), drooping of the eyelids (ptosis), stretching of the skin around the eye (involution of the dermatocalasis), and the white part of the eye becomes more visible (inferior scleral exposure).
Common:may affect up to 1 in 10 people
Affecting the eye:
- Allergic reaction in the eye
- Tired eyes
- Sensitivity to light
- Darkening of the skin around the eye
- Darkening of the eyelashes
- Pain
- Feeling of a foreign body in the eye
- Sticky eyes
- Darkening of the iris
- Difficulty seeing clearly
- Irritation
- Burning
- Inflammation, redness, and itching of the eyelids
- Tears
- Dryness
- Worsening of vision
- Blurred vision
- Inflammation of the transparent layer covering the surface of the eye
- Small epithelial erosions in the eye, with or without inflammation.
Affecting the body:
- Headaches
- Increased values in some blood tests that indicate how the liver works
- High blood pressure.
Uncommon:may affect up to 1 in 100 people
Affecting the eye:
- Cystoid macular edema (inflammation of the retina inside the eye that leads to worsening of vision)
- Inflammation inside the eye
- Retinal hemorrhage
- Swollen eyelids
- Eye twitching
- Retraction of the eyelid, moving away from the eye surface
- Redness of the skin around the eye
Affecting the body:
- Nausea
- Dizziness
- Weakness
- Hair growth around the eye
Frequency not known:cannot be estimated from the available data
Affecting the eye:
- Eye discomfort
Affecting the body:
- Asthma
- Worsening of asthma
- Worsening of a lung disease called chronic obstructive pulmonary disease (COPD)
- Breathing difficulties
- Symptoms of an allergic reaction (inflammation, redness of the eye, and skin rash)
- Discoloration of the skin (periocular)
Other side effects reported with phosphate-containing eye drops:
In very rare cases, some patients with severe damage to the transparent layer on the front of the eye (the cornea) have developed cloudy spots on the cornea due to calcium accumulation during treatment.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this package leaflet. You can also report side effects directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of VIZIBIM
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label of the bottle and on the carton after ‘EXP’. The expiry date is the last day of the month shown.
This medicine does not require any special storage conditions.
You should discard the bottle after four weeks of first opening, even if there is still liquid left in it. This will help prevent infections. To remind you, write the date you opened it on the space provided on the carton.
Do not use this medicine if you notice that the security seal of the bottle is broken before using it for the first time.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Contents of the pack and other information
Composition of VIZIBIM
- The active substance is bimatoprost. One ml of solution contains 0.3 mg of bimatoprost.
- The other ingredients are sodium chloride, disodium phosphate heptahydrate, citric acid monohydrate, and water for injections. It may contain small amounts of hydrochloric acid or sodium hydroxide to maintain the normal level of acidity (pH levels).
Appearance of VIZIBIM and contents of the pack
VIZIBIM is a clear and colorless eye drops solution, presented in a 5 ml low-density polyethylene (LDPE) bottle with a white and opaque Novelia nozzle (high-density polyethylene [HDPE] and silicone) with a blue tip, and sealed with a white HDPE cap.
The following pack sizes are available: boxes containing 1 and 3 bottles of 3 ml solution.
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer:
Marketing Authorization Holder:
BAUSCH + LOMB IRELAND LIMITED
3013 Lake Drive
Citywest Business Campus
Dublin 24, D24PPT3
Ireland
Manufacturer:
Pharmathen S.A,
Dervenakion 6, Pallini Attikis
15351 Greece
Or
EXCELVISION
27 st. La Lombardière
Zl La Lombardière
07100 ANNONAY
France
You can obtain further information on this medicine by contacting the local representative of the marketing authorization holder.
Local Representative in Spain
Bausch & Lomb S.A.
Avda. Valdelaparra, nº 4, 28018 Alcobendas
Madrid
Tel: 91 – 657 63 00
Date of last revision of this package leaflet: November 2022
Detailed information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/.
- Country of registration
- Average pharmacy price13.24 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to VIZIBIM 0.3 mg/ml EYE DROPS SOLUTIONDosage form: EYEDROP, 0.3 mg/mlActive substance: bimatoprostManufacturer: Tiedra Farmaceutica S.L.Prescription requiredDosage form: EYE DROP, 0.3 mg/mlActive substance: bimatoprostManufacturer: Sifi S.P.A.Prescription requiredDosage form: EYEDROP, 0.3 mg/mlActive substance: bimatoprostManufacturer: Laboratorio Stada S.L.Prescription required
Online doctors for VIZIBIM 0.3 mg/ml EYE DROPS SOLUTION
Discuss questions about VIZIBIM 0.3 mg/ml EYE DROPS SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















