

Bimifree

Ask a doctor about a prescription for Bimifree

How to use Bimifree
Leaflet attached to the packaging: patient information
Bimifree, 0.3 mg/ml, eye drops, solution
Bimatoprost
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, so you can read it again if you need to.
- If you have any doubts, consult your doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor or pharmacist. See section 4.
Table of contents of the leaflet
- 1. What is Bimifree and what is it used for
- 2. Important information before using Bimifree
- 3. How to use Bimifree
- 4. Possible side effects
- 5. How to store Bimifree
- 6. Contents of the pack and other information
1. What is Bimifree and what is it used for
Bimifree is a medicine used in glaucoma. It belongs to a group of medicines called prostamides.
Bimifree is used to lower high pressure in the eye. This medicine can be used alone or with other eye drops called beta-adrenergic blockers, which also lower the pressure inside the eye.
The eye contains a clear, watery fluid that nourishes the inside of the eye. This fluid is constantly drained from the eye, and new fluid is produced to replace it. If it is not drained quickly enough, the pressure in the eye increases. The action of this medicine is to increase the amount of fluid drained, and consequently, to lower the pressure inside the eye. If left untreated, high pressure can lead to a disease called glaucoma and eventually damage vision.
The medicine does not contain preservatives.
2. Important information before using Bimifree
When not to use Bimifree
- if the patient is allergic to bimatoprost or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Before starting to use Bimifree, the patient should discuss it with their doctor or pharmacist if:
- the patient has breathing difficulties
- the patient has liver or kidney problems
- the patient has had cataract surgery in the past
- the patient has low blood pressure or a slow heart rate
- the patient has had a viral infection or inflammation in the eye.
During treatment with Bimifree, the medicine may cause loss of fat tissue around the eye, which can lead to deepening of the eyelid fold, sunken eyes, drooping eyelids (ptosis), skin tension around the eye (involution caused by dermatochalasia), and increased visibility of the lower white part of the eye (visibility of the lower part of the sclera). These changes are usually mild, but if they are pronounced, they can affect the field of vision. The changes may disappear after discontinuation of treatment with Bimifree.
Bimifree may also cause darkening and excessive growth of eyelashes, as well as darkening of the skin around the eyelid. It may also darken the color of the iris. These changes can be permanent. The changes may be more noticeable if only one eye is being treated.
Children and adolescents
Bimifree has not been studied in children and adolescents under 18 years of age, so it should not be used in children and adolescents under 18 years of age.
Bimifree and other medicines
The patient should tell their doctor or pharmacist about all medicines they are taking, have recently taken, or plan to take.
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult their doctor or pharmacist before using any medicine.
Bimifree may pass into breast milk, so breastfeeding should be avoided during treatment with this medicine.
Driving and using machines
After administering Bimifree, vision may be blurred for a short time. The patient should not drive or operate machines until their vision has improved.
Bimifree contains phosphates
The medicine contains 0.95 mg of phosphates per milliliter. In patients with severe damage to the clear, front part of the eye (cornea), phosphates may, in very rare cases, cause corneal clouding due to calcium deposition during treatment.
3. How to use Bimifree
This medicine should always be used as directed by the doctor. If in doubt, the patient should consult their doctor or pharmacist.
Bimifree should only be used in the eyes. The recommended dose is one drop of Bimifree in the evening, once a day, in each eye that needs treatment.
If the patient is using Bimifree and other eye drops at the same time, they should wait at least 5 minutes between administering Bimifree and the other medicine. Eye ointments should be used last.
The medicine should not be used more than once a day, as this may reduce the effectiveness of the treatment.
Bimifree is a sterile solution that does not contain preservatives. See section 6. What Bimifree looks like and what the pack contains.
Before administering eye drops:
- When using the bottle for the first time, before administering the drops, the patient should test the bottle with the dropper by slowly squeezing it until one drop is released from the bottle. This should be done away from the eye.
- If the patient is sure they can administer a single drop, they should take the most comfortable position for administration (they can sit, lie on their back, or stand in front of a mirror).
Instructions for use:
- 1. Before administering the medicine, the patient should wash their hands thoroughly.
- 2. If the packaging or bottle is damaged, the medicine should not be used.
- 3. Before using the drops for the first time, the patient should unscrew the cap after making sure the tamper-evident ring on the cap is intact. When unscrewing, a slight resistance will be felt until the ring breaks ( figure 1.).
- 4. If the tamper-evident ring is loose, it should be removed before using the medicine, as it may fall into the eye and cause injury.
- 5. The patient should tilt their head back and gently pull the lower eyelid down with their finger to form a "pocket" between the eye and the eyelid ( figure 2.). The patient should avoid touching the tip of the dropper with the eye, eyelids, or fingers.
- 6. The patient should administer one drop by slowly squeezing the bottle ( figure 3.). The bottle should be gently squeezed in the middle, so the drop gets into the patient's eye. The patient should remember that there may be a few seconds' delay between squeezing the bottle and the drop being released. The patient should not squeeze the bottle too hard. If in doubt about using the medicine, the patient should consult their doctor, pharmacist, or nurse.
- 7. After administering the medicine, the patient should press the tear duct (the corner of the eye near the nose) with their finger for at least 2 minutes and keep their eye (eyes) closed during this time. This will ensure the medicine gets into the eye and reduce the amount of medicine that gets into the rest of the body through the tear duct.
- 8. If the doctor recommends using drops in the other eye, the patient should repeat steps 5, 6, and 7.
- 9. After use and before closing the bottle, the patient should shake the bottle downwards without touching the tip of the dropper to remove any remaining liquid from the tip of the dropper. This is necessary to ensure the next drops can be administered. Immediately after use, the bottle should be tightly closed ( figure 4.).
The patient should wipe away any excess medicine that runs down their cheek.
If a drop misses the eye, the patient should try again.
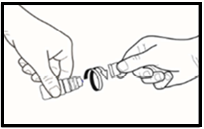
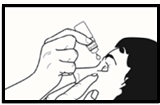
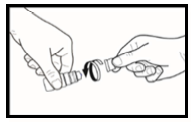
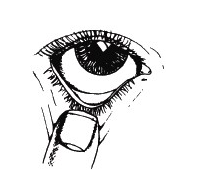
Figure 1.
Figure 2.
Figure 3.
Figure 4.
If the patient wears contact lenses, they should remove their contact lenses before using the medicine and wait at least 15 minutes before putting them back in.
Using more Bimifree than recommended
In case of using more Bimifree than recommended, it is unlikely to cause any serious harm. The next dose should be taken at the usual time.
If in doubt, the patient should consult their doctor or pharmacist.
Missing a dose of Bimifree
If a dose of Bimifree is missed, the patient should use a single drop as soon as they remember and then continue with their usual routine. The patient should not use a double dose to make up for a missed dose.
Stopping treatment with Bimifree
Bimifree should be used every day to be effective. If the patient stops using Bimifree, it may cause an increase in pressure inside the eye (intraocular pressure). Therefore, before planning to stop treatment, the patient should talk to their doctor.
If the patient has any further doubts about using this medicine, they should consult their doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Very common side effects
May affect up to 1 in 10 people
Eyelid symptoms
- mild redness (up to 24% of patients)
Symptoms around the eyes
- loss of fat tissue around the eye, which can lead to deepening of the eyelid fold, sunken eyes, drooping eyelids (ptosis), skin tension around the eye (involution caused by dermatochalasia), and increased visibility of the lower white part of the eye (visibility of the lower part of the sclera)
Common side effects
May affect up to 1 in 100 people
Eyelid symptoms
- small defects on the surface of the eye, with or without inflammation
- irritation
- itching of the eyes
- pain
- dryness
- feeling of a foreign body in the eye
- lengthening of eyelashes
- darkening of the skin around the eyes
- redness of the eyelids
Uncommon side effects
May affect up to 1 in 1000 people
Eyelid symptoms
- eye fatigue
- sensitivity to light
- darkening of the iris
- itching and swelling of the eyelids
- tearing
- swelling of the clear layer that lines the surface of the eye
- blurred vision
Whole body symptoms
- headache
- hair growth around the eyes
Side effects with unknown frequency
Eyelid symptoms
- sticky eyes
- eye discomfort
Whole body symptoms
- asthma
- worsening of asthma
- worsening of chronic obstructive pulmonary disease (COPD)
- shortness of breath
- symptoms of an allergic reaction (swelling, redness of the eye, and rash on the skin)
- dizziness
- increased blood pressure
- skin discoloration (around the eye)
In addition to these side effects observed after using bimatoprost 0.3 mg/ml in a single dose (without preservative), the following side effects were observed after using bimatoprost 0.3 mg/ml in a multi-dose form (with preservative); these side effects may also occur in patients using Bimifree 0.3 mg/ml (multi-dose packaging, without preservative):
- burning sensation in the eye
- allergic reaction in the eye
- blepharitis
- difficulty seeing clearly
- eye stickiness
- worsening of vision
- darkening of eyelashes
- retinal hemorrhage
- eye inflammation
- cystic swelling of the macula (macular edema leading to impaired vision)
- uveitis
- eyelid twitching
- eyelid contraction and pulling away from the eye surface
- nausea
- redness of the skin around the eye
- weakness
- increased liver function test results
Other side effects reported in relation to the use of eye drops containing phosphates
In patients with severe damage to the clear, front part of the eye (cornea), phosphates may, in very rare cases, cause corneal clouding due to calcium deposition during treatment.
Reporting side effects
If side effects occur, including any side effects not listed in this leaflet, the patient should tell their doctor or pharmacist. Side effects can be reported directly to the Department of Post-Marketing Surveillance of Adverse Reactions to Medicinal Products, Medical Devices, and Biocidal Products
Al. Jerozolimskie 181C
02-222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help gather more information on the safety of the medicine.
5. How to store Bimifree
There are no special precautions for storing the medicine.
The medicine should be stored out of sight and reach of children.
The medicine should not be used after the expiry date stated on the carton and bottle. The expiry date refers to the last day of the month stated.
The inscription on the packaging after the abbreviation EXP means the expiry date, and after the abbreviation Lot means the batch number.
After opening the bottle - store for 90 days at a temperature below 25°C.
The bottle should be discarded after 90 days from its first opening.
Medicines should not be disposed of via wastewater or household waste. The patient should ask their pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the pack and other information
What Bimifree contains
- The active substance of the medicine is bimatoprost. Each milliliter of solution contains 0.3 mg of bimatoprost. Each bottle contains 3 ml of solution - at least 80 drops. One drop (approximately 29.4 microliters) of solution contains approximately 8.82 micrograms of bimatoprost.
- The other ingredients are: citric acid monohydrate, disodium phosphate dodecahydrate, sodium chloride, hydrochloric acid, diluted (to adjust pH), water for injection.
What Bimifree looks like and what the pack contains
Bimifree is a clear, colorless solution.
The medicine is available in white bottles with a capacity of 5 ml (LDPE) containing 3 ml of solution, with a multi-dose dropper (HDPE) that prevents contamination of the solution with bacteria thanks to a system consisting of a silicone membrane and air filtration entering the bottle, with a cap made of HDPE with a tamper-evident ring, in a cardboard box.
Packaging:
1 bottle with a capacity of 5 ml containing 3 ml of solution.
3 bottles with a capacity of 5 ml containing 3 ml of solution each.
Not all pack sizes may be marketed.
Marketing authorization holder
Pharmaceutical Works POLPHARMA S.A.
Pelplińska 19, 83-200 Starogard Gdański
phone: +48 22 364 61 01
Manufacturer
Rafarm S.A.
Thesi Pousi Xatzi Agiou Louka
Paiania, 190 02
Greece
Date of last revision of the leaflet:
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterRafarm S.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to BimifreeDosage form: Drops, 0.3 mg/mlActive substance: bimatoprostPrescription requiredDosage form: Gel, 0.1 mg/gActive substance: bimatoprostManufacturer: Laboratoire Unither Laboratoires THEAPrescription requiredDosage form: Drops, 0.3 mg/mlActive substance: bimatoprostManufacturer: Excelvision Pharmathen S.A.Prescription required
Alternatives to Bimifree in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Bimifree in Spain
Alternative to Bimifree in Ukraine
Online doctors for Bimifree
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Bimifree – subject to medical assessment and local rules.














