
VISCOTEÍNA 50 mg/ml ORAL SOLUTION

How to use VISCOTEÍNA 50 mg/ml ORAL SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
PACKAGE LEAFLET: INFORMATION FOR THE USER
Viscoteína 50 mg/ml oral solution
Carbocisteine
Read all of this leaflet carefully because it contains important information for you.
This medicine can be obtained without a prescription. Nevertheless, to get the best results, it should be used properly.
- Keep this leaflet, as you may need to read it again.
- If you need advice or more information, consult your pharmacist.
- If symptoms worsen or persist after 5 days, or if fever, skin rash, persistent headache, or sore throat occurs, you should consult a doctor.
- If you consider that any of the adverse effects you are suffering from is serious or if you notice any adverse effect not mentioned in this leaflet, inform your doctor or pharmacist.
Contents of the package leaflet:
- What is Viscoteína and what is it used for
- Before taking Viscoteína
- How to take Viscoteína
- Possible side effects
- Storage of Viscoteína
- Additional information
1. What is Viscoteína and what is it used for
Viscoteína belongs to a group of medicines called mucolytics, and it is used to fluidify excessive and/or thick bronchial secretions, facilitating their elimination.
2. Before taking Viscoteína
Do not take Viscoteína:
- If you are allergic (hypersensitive) to carbocisteine and its derivatives, or to any of the other components of Viscoteína.
- If you have a stomach or duodenal ulcer.
- This medicine should not be administered to children under 2 years of age.
- If you are asthmatic or have severe respiratory failure.
Be careful with Viscoteína:
- If you have severe liver or kidney failure
- In elderly patients, who are especially sensitive to the adverse effects of carbocisteine.
- During the first few days, it is normal for expectoration to increase, but if symptoms persist for more than 5 days or worsen, you should consult your doctor.
Use of other medicines:
Inform your doctor or pharmacist if you are using or have recently used other medicines, including those purchased without a prescription.
Consult your doctor before taking Viscoteína if you are taking cough medicines or those that reduce bronchial secretions.
Pregnancy and breastfeeding
Consult your doctor or pharmacist before using any medicine.
This medicine should not be taken during pregnancy or breastfeeding.
IMPORTANT FOR WOMEN
If you are pregnant or think you may be pregnant, consult your doctor before taking this medicine. The consumption of medicines during pregnancy can be dangerous for the embryo or fetus and should be monitored by your doctor.
Driving and using machines
No interference has been described regarding driving and/or using machines while taking Viscoteína.
Important information about some of the components of Viscoteína
This medicine contains sucrose. If your doctor has indicated that you have an intolerance to certain sugars, consult with them before taking this medicine.
Patients with diabetes mellitus should note that this medicine contains 1.5 g of sucrose per 5 ml.
It may cause allergic reactions (possibly delayed) because it contains methyl parahydroxybenzoate, sodium salt (E-219) and propyl parahydroxybenzoate, sodium salt (E-217).
Patients on low-sodium diets should note that this medicine contains 34.75 mg of sodium per 5 ml dose and 104.24 mg of sodium per 15 ml dose.
3. How to take Viscoteína
Consult your doctor or pharmacist if you have any doubts.
This medicine is administered orally, alone or diluted in water or another liquid, and preferably before meals. The normal dose is:
- Adults and children over 12 years: 15 ml (750 mg of carbocisteine) three times a day.
- Children from 5 to 12 years: 5 ml (250 mg of carbocisteine) three times a day.
- Children from 2 to 5 years: 2.5 ml (125 mg of carbocisteine) three times a day.
It is recommended to drink plenty of liquid throughout the day.
Instructions for the correct administration of the preparation
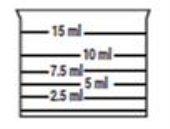
In order to ensure correct dosing and given its use in pediatrics, it is recommended to perform dosing using the measuring cup included in the presentation, which has the following measures marked: 2.5 ml, 5 ml, 7.5 ml, 10 ml, and 15 ml.
Place the measuring cup on a flat surface and at eye level. Fill it with the solution up to the line that indicates your dose.
After use, wash the measuring cup with water.
If symptoms worsen or persist after 5 days, or if fever, skin rash, persistent headache, or sore throat occurs, you should consult a doctor.
If you take more Viscoteína than you should:
Accidental ingestion of very high doses can cause hypersensitivity reactions, nausea, vomiting, diarrhea, headache, gastrointestinal bleeding, etc.
If you have taken more Viscoteína than you should, consult your doctor or pharmacist immediately, or call the Toxicology Information Service, phone: 91 562 0420, indicating the medicine and the amount ingested.
If you forget to take Viscoteína:
Do not take a double dose to make up for forgotten doses.
If you have any other doubts about the use of this product, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, Viscoteína can have side effects, although not everyone gets them.
The side effects observed are described below according to their frequency of presentation: Very common (affects more than 1 in 10 patients), Common (affects between 1 and 10 in 100 patients), Uncommon (affects between 1 and 10 in 1,000 patients), Rare (affects between 1 and 10 in 10,000 patients), Very rare (affects less than 1 in 10,000 patients).
Common side effects (affects between 1 and 10 in 100 patients):
Digestive disorders (gastric discomfort, nausea, diarrhea) may occur, especially at high doses, which usually disappear with a reduction in the administered dose.
Rare side effects (affects between 1 and 10 in 10,000 patients):
Hypersensitivity reactions (allergic reactions), accompanied by urticaria (appearance of hives and itching), headache, skin rash.
Very rare side effects (affects less than 1 in 10,000 patients):
Bronchospasm (sudden sensation of difficulty breathing as occurs in asthma). In these cases, it is advised to suspend treatment and consult a doctor.
Reporting of side effects
If you experience any type of side effect, consult your doctor or pharmacist, even if it is a possible side effect not listed in this leaflet. You can also report them directly through the Spanish Medicines and Health Products Agency's website: www.notificaRAM.es. By reporting side effects, you can contribute to providing more information on the safety of this medicine.
5. Storage of Viscoteína
Keep out of the reach and sight of children.
Store below 30°C.
Do not use Viscoteína after the expiration date shown on the packaging after CAD. The expiration date is the last day of the month indicated.
Medicines should not be disposed of through wastewater or household waste. Deposit the packaging and medicines you no longer need at the SIGRE point in the pharmacy. In case of doubt, ask your pharmacist how to dispose of the packaging and medicines you no longer need. This way, you will help protect the environment.
6. Additional information
Composition of Viscoteína:
- The active ingredient is carbocisteine. Each ml of oral solution contains 50 mg of carbocisteine.
- The other components are: sucrose, sodium saccharin, methyl parahydroxybenzoate, sodium salt (E-219), propyl parahydroxybenzoate, sodium salt (E-217), caramel color (E-150), caramel flavor, sodium hydroxide for pH adjustment, purified water.
Appearance of the product and packaging content:
Viscoteína is an oral solution that comes in a glass bottle with a polypropylene/polyethylene high-density (PP/HDPE) cap with a child-proof closure, containing 200 ml of oral solution and a measuring cup.
The measuring cup has the following measures marked: 2.5 ml, 5 ml, 7.5 ml, 10 ml, and 15 ml.
Marketing authorization holder
Faes Farma, S.A.
Autonomia Etorbidea, 10
48940 Leioa (Bizkaia)
Spain
Manufacturer
Faes Farma, S.A.
Parque Científico y Tecnológico de Bizkaia
Ibaizabal Bidea, Edificio 901
48160 Derio (Bizkaia)
Spain
This leaflet was approved in: February 2025
Detailed and updated information about this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to VISCOTEÍNA 50 mg/ml ORAL SOLUTIONDosage form: ORAL SOLUTION/SUSPENSION, 2 g carbocysteine / 100 mlActive substance: carbocisteineManufacturer: Almirall S.A.Prescription requiredDosage form: ORAL SOLUTION/SUSPENSION, 50 mg carbocysteine / mlActive substance: carbocisteineManufacturer: Laboratorios Cinfa S.A.Prescription not requiredDosage form: ORAL SOLUTION/SUSPENSION, 750 mgActive substance: carbocisteineManufacturer: Laboratorios Cinfa S.A.Prescription not required
Online doctors for VISCOTEÍNA 50 mg/ml ORAL SOLUTION
Discuss questions about VISCOTEÍNA 50 mg/ml ORAL SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















