
VFEND 40 mg/ml POWDER FOR ORAL SUSPENSION


How to use VFEND 40 mg/ml POWDER FOR ORAL SUSPENSION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
VFEND40mg/ml powder for oral suspension
voriconazole
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is VFEND and what is it used for.
- What you need to know before you take VFEND.
- How to take VFEND.
- Possible side effects.
- Storage of VFEND.
- Contents of the pack and further information.
1. What is VFEND and what is it used for
VFEND contains the active substance voriconazole. VFEND is an antifungal medicine. It works by killing or preventing the growth of fungi that cause infections.
It is used for the treatment of patients (adults and children of 2 years or older) with:
- invasive aspergillosis (a type of fungal infection caused by Aspergillus sp),
- candidaemia (another type of fungal infection caused by Candida sp) in non-neutropenic patients (patients who do not have an abnormally low count of white blood cells),
- serious invasive infections caused by Candida sp, when the fungus is resistant to fluconazole (another antifungal medicine),
- serious fungal infections caused by Scedosporium spor Fusarium sp(two different species of fungi).
VFEND is used in patients with serious fungal infections that may be life-threatening.
Prevention of fungal infections in bone marrow transplant recipients with high risk.
This medicine should only be used under medical supervision.
2. What you need to know before you take VFEND
Do not take VFEND:
if you are allergic to voriconazole or any of the other ingredients of this medicine (listed in section 6).
It is very important that you inform your doctor or pharmacist if you are using, have recently used or might use any other medicines or herbal products.
During treatment with VFEND, you must not take the following medicines:
- Terfenadine (used for allergy).
- Astemizole (used for allergy).
- Cisapride (used for digestive problems).
- Pimozide (used for mental disorders).
- Quinidine (used for heart rhythm disorders).
- Ivabradine (used for symptoms of chronic heart failure).
- Rifampicin (used for the treatment of tuberculosis).
- Efavirenz (used for the treatment of HIV) at doses of 400 mg and above once daily.
- Carbamazepine (used to treat seizures).
- Phenobarbital (used for severe insomnia and seizures).
- Ergot alkaloids (e.g. ergotamine, dihydroergotamine; used for migraine).
- Sirolimus (used in patients who have received a transplant).
- Ritonavir (used for the treatment of HIV) at doses of 400 mg and above twice daily.
- St. John's Wort (hypericum, herbal product).
- Naloxegol (used to treat constipation caused by opioid painkillers [e.g. morphine, oxycodone, fentanyl, tramadol, codeine]).
- Tolvaptan (used to treat low sodium levels in the blood or to slow the worsening of kidney function in patients with polycystic kidney disease).
- Lurasidone (used to treat depression).
- Venetoclax (used to treat patients with chronic lymphocytic leukaemia [CLL]).
Warnings and precautions
Consult your doctor, pharmacist, or nurse before starting VFEND if:
- you have had an allergic reaction to other azoles.
- you have or have had liver disease. If you have liver disease, your doctor may prescribe a lower dose of VFEND. Your doctor should also monitor your liver function with blood tests while you are taking VFEND.
- you know that you have cardiomyopathy, irregular heartbeats, slow heart rate, or an abnormality in your electrocardiogram (ECG) called "QTc prolonged syndrome".
You should avoid any exposure to the sun and sunlight during treatment. It is important that you cover exposed areas and use a sunscreen with a high sun protection factor (SPF), as you may be more sensitive to the UV rays of the sun. This sensitivity may be increased further by the use of other medicines that make your skin more sensitive to sunlight, such as methotrexate. These precautions also apply to children.
While taking VFEND:
- tell your doctor if you get
- sunburn
- severe skin rash or blisters
- bone pain
If you develop skin disorders such as those described above, your doctor may refer you to a dermatologist, who may consider it important to examine you regularly. There is a small chance that you may develop skin cancer with long-term use of VFEND.
If you develop signs of "adrenal insufficiency" where your adrenal glands do not produce enough of certain steroid hormones, such as cortisol, which can cause symptoms such as: chronic or prolonged fatigue, muscle weakness, loss of appetite, weight loss, abdominal pain, tell your doctor.
If you develop signs of "Cushing's syndrome" where your body produces too much cortisol, which can cause symptoms such as: weight gain, hump of fat between the shoulders, rounded face, darkening of the skin of the abdomen, thighs, breasts, and arms, thin skin, easy bruising, high blood sugar levels, excessive hair growth or excessive sweating, tell your doctor.
Your doctor should monitor your liver and kidney function with blood tests.
Children and adolescents
VFEND should not be given to children under 2 years of age.
Other medicines and VFEND
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines.
Some medicines, when used at the same time as VFEND, may affect the action of VFEND or VFEND may affect the action of other medicines.
Tell your doctor if you are using the following medicines, as concurrent treatment with VFEND should be avoided if possible:
- Ritonavir (used for the treatment of HIV) at doses of 100 mg twice daily.
- Glasdegib (used for the treatment of cancer); if you need to use both medicines, your doctor will monitor your heart rate frequently.
Tell your doctor if you are using any of the following medicines, as concurrent treatment with VFEND should be avoided whenever possible, and a dose adjustment of voriconazole may be necessary:
- Rifabutin (used for the treatment of tuberculosis). If you are already being treated with rifabutin, your doctor will need to monitor your blood count and the side effects of rifabutin.
- Phenytoin (used to treat epilepsy). If you are already being treated with phenytoin, your doctor will need to monitor the concentration of phenytoin in your blood during your treatment with VFEND and may need to adjust your dose.
Tell your doctor if you are taking any of the following medicines, as you may need a dose adjustment or monitoring to check that these medicines and/or VFEND are still working properly:
- Warfarin and other anticoagulants (e.g. phenprocoumon, acenocoumarol; used to reduce blood clotting).
- Ciclosporin (used in patients who have received a transplant).
- Tacrolimus (used in patients who have received a transplant).
- Sulphonylureas (e.g. tolbutamide, glipizide, and gliburide) (used for diabetes).
- Statins (e.g. atorvastatin, simvastatin) (used to reduce cholesterol levels).
- Benzodiazepines (e.g. midazolam, triazolam) (used for severe insomnia and stress).
- Omeprazole (used for the treatment of stomach ulcers).
- Oral contraceptives (if you take VFEND while using oral contraceptives, you may experience side effects such as nausea and menstrual disorders).
- Vinca alkaloids (e.g. vincristine and vinblastine) (used to treat cancer).
- Tyrosine kinase inhibitors (e.g. axitinib, bosutinib, cabozantinib, ceritinib, cobimetinib, dabrafenib, dasatinib, nilotinib, sunitinib, ibrutinib, ribociclib) (used to treat cancer).
- Tretinoin (used to treat leukaemia).
- Indinavir and other HIV protease inhibitors (used to treat HIV infection).
- Non-nucleoside reverse transcriptase inhibitors (e.g. efavirenz, delavirdine, and nevirapine) (used to treat HIV infection) (some doses of efavirenz MUST NOT be taken at the same time as VFEND).
- Methadone (used to treat heroin addiction).
- Alfentanil, fentanyl, and other short-acting opioids such as sufentanil (painkillers used for operations).
- Oxycodone and other long-acting opioids such as hydrocodone (used to treat moderate to severe pain).
- Non-steroidal anti-inflammatory medicines (e.g. ibuprofen, diclofenac) (used to treat pain and inflammation).
- Fluconazole (used to treat fungal infections).
- Everolimus (used to treat advanced kidney cancer and in patients who have received a transplant).
- Letermovir (used to prevent cytomegalovirus (CMV) infection after a bone marrow transplant).
- Ivacaftor: used to treat cystic fibrosis.
- Flucloxacillin (antibiotic used against bacterial infections).
Pregnancy and breast-feeding
Do not take VFEND during pregnancy unless your doctor has told you to. Women of childbearing age taking VFEND should use effective contraception. Contact your doctor immediately if you become pregnant while taking VFEND.
If you are pregnant or breast-feeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
Driving and using machines
VFEND may cause blurred vision or sensitivity to light. If this happens, do not drive or use tools or machines and tell your doctor.
VFEND contains sucrose
This medicine contains sucrose. If your doctor has told you that you have an intolerance to some sugars, consult them before taking this medicine.
Patients with diabetes mellitus should note that this medicine contains 0.54 g of sucrose per ml of suspension.
It may cause tooth decay.
VFEND contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per 5 ml of suspension; this is essentially "sodium-free".
VFEND contains benzonic acid/sodium salt
This medicine contains 12 mg of benzonic acid/sodium salt (E211) in each 5 ml dose.
3. How to take VFEND
Follow the administration instructions for this medication exactly as indicated by your doctor. In case of doubt, consult your doctor or pharmacist again.
Your doctor will determine the dose based on your weight and the type of infection you have.
The recommended dose in adults (including elderly patients) is as follows:
Oral Suspension | ||
Patients with weight equal to or greater than 40 kg | Patients with weight less than 40 kg | |
Dose during the first 24hours (loading dose) | 10 ml (400 mg) every 12 hours during the first 24 hours | 5 ml (200 mg) every 12 hours during the first 24 hours |
Dose after the first 24hours (maintenance dose) | 5 ml (200 mg) twice a day | 2.5 ml (100 mg) twice a day |
Depending on your response to treatment, your doctor may increase the daily dose to 7.5 ml (300 mg) twice a day.
Your doctor may decide to reduce the dose if you have mild to moderate cirrhosis.
Use in children and adolescents
The recommended dose in children and adolescents is as follows:
Oral Suspension | ||
Children from 2 to less than 12 years and adolescents from 12 to 14 years who weigh less than 50 kg | Adolescents from 12 to 14 years who weigh 50 kg or more; and all adolescents over 14 years | |
Dose during the first 24hours (loading dose) | Your treatment should be started with an infusion | 10 ml (400 mg) every 12 hours during the first 24 hours |
Dose after the first 24hours (maintenance dose) | 0.225 ml/kg (9 mg/kg) twice a day (maximum dose of 8.75 ml [350 mg] twice a day) | 5 ml (200 mg) twice a day |
Depending on your response to treatment, your doctor may increase or decrease the daily dose.
Take the suspension at least one hour before or two hours after meals.
If you or your child are taking VFEND to prevent fungal infections, your doctor may stop the administration of VFEND if you or your child experience treatment-related adverse effects.
VFEND suspension should not be mixed with any other medication. The suspension should not be diluted further with water or other liquids.
Instructions for preparing the suspension
It is recommended that your pharmacist prepare the VFEND suspension before giving it to you.
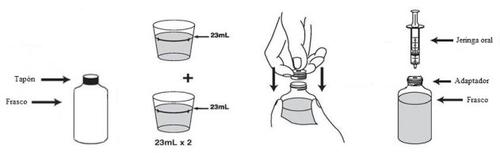
VFEND suspension is reconstituted if it has a liquid form. If it appears as a dry powder, the oral suspension must be reconstituted following the instructions detailed below:
- Gently tap the bottle to loosen the powder.
- Remove the cap.
- Add to the bottle 2 measuring cups (the measuring cup is included in the box) of water (for a total of 46 ml). Fill the measuring cup to the top of the marked line and then add the measured water to the bottle. You should always add a total of 46 ml of water, regardless of the dose you need to take.
- Put the cap back on and shake the bottle vigorously for about 1 minute. After reconstitution, the total volume of the suspension should be 75 ml.
- Remove the cap. Press the adapter into the neck of the bottle (as shown in the following drawing). The adapter is provided to fill the oral syringe with the medication from the bottle. Put the cap back on the bottle.
- Write the expiration date of the reconstituted suspension on the bottle label (the validity period of the reconstituted suspension is 14 days). Any remaining suspension should be discarded after this date.
Instructions for use:
Your pharmacist should explain how to measure the medication using the multidose oral syringe included in the package. Please read the following instructions before using VFEND suspension.
- Shake the closed bottle with the reconstituted suspension for about 10 seconds before use. Remove the cap.
- With the bottle upside down, placed on a flat surface, insert the end of the oral syringe into the adapter.
- Keeping the syringe in place, turn the bottle upside down. Slowly pull the syringe plunger to the graduated mark corresponding to your dose.
- If you see large bubbles, slowly press the syringe plunger. This will cause the medication to return to the bottle. Repeat step 3 again.
- Put the bottle upside down with the syringe in place. Remove the syringe from the bottle.
- Place the end of the syringe in your mouth. Point the syringe towards the inside of your cheek. Press the syringe plunger SLOWLY. Do not expel the medication quickly. If you are going to administer the medication to a child, make sure the child is sitting or held upright before giving them the medication.
- Put the cap back on the bottle, leaving the adapter in place. Wash the syringe following the instructions that appear below.
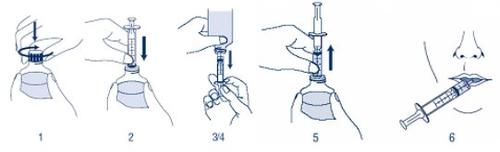
Cleaning and storage of the syringe:
- You should wash the syringe after each use. Remove the plunger from the syringe and wash the two parts with warm soapy water. Then, rinse with water.
- Dry the two parts. Put the plunger back in the syringe. Store it in a clean and safe place along with the medication.
If you take more VFEND than you should
If you take more suspension than prescribed (or if someone else takes your suspension), you should seek medical attention immediately or go to the nearest hospital emergency department. Bring your VFEND suspension bottle with you. You may notice an abnormal sensitivity to light as a result of taking more VFEND than you should.
If you forget to take VFEND
It is important to take the VFEND suspension regularly at the same time of day. If you forget to take a dose, take the next one when it is due. Do not take a double dose to make up for missed doses.
If you interrupt treatment with VFEND
It has been shown that correctly following the indicated posology, administering each dose at the right time, can significantly increase the effectiveness of this medication. Therefore, unless your doctor tells you to interrupt treatment, it is essential that you continue taking VFEND correctly as indicated above.
Continue taking VFEND until your doctor tells you to stop. Do not stop treatment prematurely, as the infection may not be cured. Patients with compromised immune systems or complicated infections may require longer treatments to prevent the infection from recurring.
When your doctor stops treatment with VFEND, you should not experience any effects from stopping it.
If you have any further questions about the use of this medication, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medications, this medication can cause side effects, although not everyone will experience them.
If they appear, it is most likely that they will be mild and transient. However, some may be serious and require medical attention.
Severe side effects - Stop taking VFEND and see a doctor immediately
- Skin rash.
- Jaundice, alterations in blood tests to control liver function.
- Pancreatitis.
Other side effects
Very common: may affect more than 1 in 10 people
- Visual disturbance (change in vision, such as blurred vision, color vision disturbances, abnormal intolerance to visual perception of light, color blindness, eye disorder, visual halo, night blindness, oscillating vision, sparkles, visual aura, decreased visual acuity, visual clarity, partial loss of usual field of vision, spots in the field of vision).
- Fever.
- Skin rash.
- Nausea, vomiting, and diarrhea.
- Headache.
- Swelling of the limbs.
- Stomach pain.
- Difficulty breathing.
- Elevated liver enzymes.
Common: may affect up to 1 in 10 people
- Sinusitis, gum inflammation, chills, weakness.
- Low count, including severe, of some types of red or white blood cells in the blood, low platelet count that helps blood clot.
- Low blood sugar levels, low potassium levels in the blood, low sodium levels in the blood.
- Anxiety, depression, confusion, agitation, insomnia, hallucinations.
- Seizures, tremors or uncontrolled muscle movements, tingling or abnormal sensations in the skin, increased muscle tone, somnolence, dizziness.
- Eye hemorrhage.
- Heart rhythm problems, including very fast or very slow heartbeat, fainting.
- Hypotension, inflammation of the veins (which may be associated with the formation of blood clots).
- Acute difficulty breathing, chest pain, swelling of the face (mouth, lips, and around the eyes), fluid retention in the lungs.
- Constipation, indigestion, lip inflammation.
- Jaundice (yellowish skin color), liver inflammation and liver damage.
- Skin rashes, which can be severe, with blisters and peeling, characterized by a flat, red area covered with small bumps that merge, skin redness.
- Itching.
- Hair loss.
- Back pain.
- Kidney failure, blood in urine, alterations in kidney function tests.
- Sunburn or severe skin reactions after exposure to light or sun.
- Skin cancer.
Uncommon: may affect up to 1 in 100 people
- Flu-like symptoms, irritation and inflammation of the gastrointestinal tract, inflammation of the gastrointestinal tract that causes antibiotic-associated diarrhea, inflammation of the lymphatic vessels.
- Inflammation of the tissue that lines the inner wall of the abdomen and covers the abdominal organs.
- Lymph node enlargement (sometimes painful), bone marrow failure, increased eosinophils.
- Decreased adrenal gland function, underactive thyroid gland.
- Abnormal brain function, symptoms similar to Parkinson's disease, nerve damage causing numbness, pain, tingling, or burning in the hands or feet.
- Problems with balance or coordination.
- Brain swelling.
- Double vision, serious eye diseases, such as: eye and eyelid pain and inflammation, abnormal eye movements, optic nerve damage that causes vision disturbances, inflammation of the optic papilla.
- Decreased sensitivity to touch.
- Alteration of the sense of taste.
- Hearing difficulties, ringing in the ears, vertigo.
- Inflammation of certain internal organs, pancreas, and duodenum, swelling and inflammation of the tongue.
- Liver enlargement, liver failure, gallbladder disease, gallstones.
- Joint inflammation, inflammation of the veins under the skin (which may be associated with the formation of a blood clot).
- Kidney inflammation, protein in the urine, kidney damage.
- Very high or extra heartbeats, sometimes with erratic electrical impulses.
- Abnormal electrocardiogram (ECG).
- High cholesterol in the blood, high urea in the blood.
- Skin allergic reactions (sometimes severe), such as a potentially life-threatening skin disease that causes blisters and painful sores on the skin and mucous membranes, especially in the mouth, skin inflammation, hives, skin redness and irritation, reddish-purple skin color that may be caused by low platelet count, eczema.
- Reaction at the infusion site.
- Allergic reaction or exaggerated immune response.
- Inflammation of the tissue surrounding the bone.
Rare: may affect up to 1 in 1,000 people
- Overactive thyroid gland.
- Worsening of brain function as a serious complication of liver disease.
- Loss of most of the optic nerve fibers, corneal opacity, involuntary eye movement.
- Blisters due to photosensitivity.
- Disorder in which the immune system attacks part of the peripheral nervous system.
- Heart rhythm or conduction problems (sometimes potentially fatal).
- Potentially fatal allergic reaction.
- Alterations in blood coagulation.
- Skin allergic reactions (sometimes severe), such as rapid swelling (edema) of the dermis, subcutaneous tissue, mucosa, and submucosal layers, itchy and painful thickened skin with silvery scales, skin and mucous membrane irritation, potentially life-threatening skin disease that causes large portions of the epidermis, the outermost layer of the skin, to peel off from the layers of skin underneath.
- Small, scaly, dry patches on the skin, sometimes thick and with points or "horns".
Side effects with unknown frequency:
- Freckles and pigmented spots.
Other important side effects whose frequency is unknown, but which should be reported to the doctor immediately:
- Red, scaly patches or ring-shaped skin lesions that may be a symptom of an autoimmune disease called cutaneous lupus erythematosus.
Since VFEND has been shown to affect the liver and kidneys, your doctor should monitor liver and kidney function through blood tests. Inform your doctor if you have stomach pain or if your stools have a different consistency.
Cases of skin cancer have been reported in patients treated with VFEND for long periods.
The frequency of sunburn or severe skin reactions after exposure to light or sun was higher in children. If you or your child experience skin disorders, your doctor may refer you to a dermatologist who, after consultation, may decide that it is essential for you or your child to undergo regular follow-up. Elevated liver enzymes were also observed more frequently in children.
If any of these side effects persist or are bothersome, inform your doctor.
Reporting side effects
If you experience any type of side effect, consult your doctor, pharmacist, or nurse, even if it is a possible side effect that does not appear in this leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: www.notificaRAM.es. By reporting side effects, you can contribute to providing more information on the safety of this medication.
5. Storage of VFEND
Keep this medication out of sight and reach of children.
Do not use this medication after the expiration date that appears on the label. The expiration date is the last day of the month indicated.
Oral suspension powder: store between 2°C - 8°C (in the refrigerator) before reconstitution.
For the suspension, once reconstituted:
Do not store at a temperature above 30°C.
Do not refrigerate or freeze.
Store in the original package.
Keep the package tightly closed.
Any remaining suspension should be discarded 14 days after reconstitution.
Medications should not be thrown away through wastewater or household waste. Ask your pharmacist how to dispose of packages and medications that you no longer need. This will help protect the environment.
6. Package contents and additional information
Composition of VFEND
- The active ingredient is voriconazole. Each bottle contains 45 g of powder, which, once reconstituted with water following the instructions, results in 70 ml of suspension. One ml of the reconstituted suspension contains 40 mg of voriconazole. (see section 3 'How to take VFEND')
- The other ingredients are sucrose; colloidal silicon dioxide; titanium dioxide; xanthan gum; sodium citrate; sodium benzoate; citric acid; natural orange flavor (see section 2, VFEND 40 mg/ml oral suspension powder contains sucrose, sodium benzoate, and sodium).
Appearance of the product and package contents
VFEND is presented as a white to off-white powder for oral suspension that, once reconstituted with water, results in a white to off-white suspension with an orange flavor.
Marketing authorization holder and manufacturer
Pfizer Europe MA EEIG, Boulevard de la Plaine 17, 1050 Bruxelles, Belgium.
Manufacturer
Fareva Amboise, Zone Industrielle, 29 route des Industries, 37530 Pocé-sur-Cisse, France.
You can request more information about this medication by contacting the local representative of the marketing authorization holder.
Spain |
Pfizer, S.L. |
Tel: +34 91 490 99 00 |
Date of the last approval of this leaflet: 04/2024.
Detailed information about this medication is available on the European Medicines Agency website: http://www.ema.europa.eu.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to VFEND 40 mg/ml POWDER FOR ORAL SUSPENSIONDosage form: TABLET, UnknownActive substance: voriconazoleManufacturer: Pfizer Europe Ma EeigPrescription requiredDosage form: TABLET, UnknownActive substance: voriconazoleManufacturer: Pfizer Europe Ma EeigPrescription requiredDosage form: TABLET, UnknownActive substance: voriconazoleManufacturer: Pfizer Europe Ma EeigPrescription required
Online doctors for VFEND 40 mg/ml POWDER FOR ORAL SUSPENSION
Discuss questions about VFEND 40 mg/ml POWDER FOR ORAL SUSPENSION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















