VERRUPATCH 3.75 mg CUTANEOUS PATCHES

How to use VERRUPATCH 3.75 mg CUTANEOUS PATCHES
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Leaflet: information for the user
Verrupatch 3.75mg transdermal patches
salicylic acid
Read the entire leaflet carefully before starting to use this medicine, as it contains important information for you. Follow the administration instructions of the medicine contained in this leaflet or as indicated by your doctor or pharmacist.
|
Contents of the leaflet
- What is Verrupatch and what is it used for
- What you need to know before using Verrupatch
- How to use Verrupatch
- Possible side effects
- Storage of Verrupatch
- Package contents and additional information
1. What is Verrupatch and what is it used for
Verrupatch is a medicine in the form of transdermal patches and belongs to the group of anti-wart medicines.
It contains salicylic acid as the active ingredient, with anti-wart properties due to its keratolytic activity, which produces softening and subsequent destruction of the stratum corneum, allowing the elimination of warts.
Verrupatch is indicated for the local treatment of common warts, which usually appear on the hands or feet (plantar warts).
2. What you need to know before using Verrupatch
Do not use Verrupatch
- If you are allergic to salicylic acid or any of the other components of this medicine (listed in section 6).
- Do not apply to moles, birthmarks, warts with hair, genital warts, warts on the face or mucous membranes.
- Do not use this product on irritated or wounded skin or on any area that is infected, inflamed, or reddened.
- Do not use in children under 2 years of age.
Warnings and precautions
Consult your doctor or pharmacist before starting to use Verrupatch.
Do not ingest. For cutaneous use (exclusively on the skin).
Avoid contact with healthy skin.
Avoid contact with the eyes.
In case of accidental application to the eyes, rinse with plenty of water.
Diabetic or poorly circulated individuals should consult their doctor before using this medicine.
Children
Do not use in children between 2 and 12 years old without medical advice (see Do not use Verrupatch).
Young children may be at greater risk of side effects.
Other medicines and Verrupatch
Tell your doctor or pharmacist if you are using, have recently used, or may need to use any other medicine.
Do not use this medicine together with other keratolytics (anti-wart medicines) in the same area, as they may enhance its effect.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
Do not use Verrupatch during pregnancy, except for short-term treatment of a small wart.
Oral forms (e.g., tablets) of this class of medicine may cause side effects in the fetus. It is unknown if the same risks apply to Verrupatch when used for warts.
A decision should be made whether to discontinue breastfeeding or discontinue treatment, considering the benefit of breastfeeding for the child and the benefit of treatment for the mother.
C
Verrupatch has no or negligible influence on the ability to drive and use machines.
Verrupatch contains propylene glycol (E-1520)
This medicine contains 11.25 mg of propylene glycol in each dose unit (patch).
Propylene glycol may cause skin irritation.
3. How to use Verrupatch
Follow the administration instructions of the medicine contained in this leaflet or as indicated by your doctor or pharmacist. In case of doubt, ask your doctor or pharmacist.
For cutaneous use (on the skin).
The recommended dose is one Verrupatch patch once a day, preferably before bedtime.
Use in children
Do not use in children under 2 years of age.
In children under 12 years of age and over 2 years, do not use without medical advice and avoid prolonged use in children.
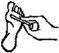
Instructions for correct administration

|
|
|
 Leave the patch on for the night (about 8 hours) and remove it in the morning. Repeat this process daily, for a maximum of 12 weeks, until the wart is eliminated. Generally, a noticeable improvement occurs during the first few days, so a complete resolution can be expected after a period of 3 to 6 weeks of treatment.
Leave the patch on for the night (about 8 hours) and remove it in the morning. Repeat this process daily, for a maximum of 12 weeks, until the wart is eliminated. Generally, a noticeable improvement occurs during the first few days, so a complete resolution can be expected after a period of 3 to 6 weeks of treatment.
Consult your doctor or pharmacist if you do not notice an improvement after 1 week, or if the warts are numerous or if an infection or inflammation occurs.
If you use more Verrupatch than you should
Overdose cases are not expected due to the mode of application of this medicine. Excessive use may cause irritation, especially on healthy skin. Use emollients if necessary.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call a medical center or the Toxicology Information Service, telephone 91 562 04 20, indicating the medicine and the amount used.
If you forget to use Verrupatch
Do not apply a double dose to make up for forgotten doses. Apply the product again at your usual time
If you stop treatment with Verrupatch
If you have any other doubts about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everyone may experience them.
Local irritation may occur if the patches come into contact with the healthy skin surrounding the wart. In this case, it is recommended to temporarily suspend treatment until the irritation disappears. When resuming treatment, make sure the patch is properly cut to only come into contact with the wart tissue.
Excessive scaling has been reported in application to open lesions; also, serious allergic reactions.
Reporting side effects
If you experience any type of side effect, consult your doctor or pharmacist, even if it is a possible side effect not listed in this leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: www.notificaRAM.es. By reporting side effects, you can contribute to providing more information on the safety of this medicine.
5. Storage of Verrupatch
Keep this medicine out of the sight and reach of children.
No special storage conditions are required.
Do not use this medicine after the expiration date shown on the package after CAD. The expiration date is the last day of the month indicated.
Do not use this medicine if you notice visible signs of deterioration in the appearance of the transdermal patches.
Medicines should not be disposed of through wastewater or household waste. Deposit the packaging and medicines you no longer need at the SIGRE point in the pharmacy. If in doubt, ask your pharmacist how to dispose of the packaging and medicines you no longer need. This will help protect the environment.
6. Package contents and additional information
Composition of Verrupatch
- The active ingredient is salicylic acid. Each patch contains 3.75 mg of salicylic acid.
- The other ingredients (excipients) are: propylene glycol (E-1520), karaya gum, polyethylene glycol 300 (Macrogol 300), and quaternium-15.
Appearance of the product and package contents
Verrupatch is presented in the form of circular transdermal patches, 6 mm in diameter, beige in color, and covered with a blue polyethylene film that retains moisture, exerting an occlusive effect.
Each package contains 20 patches arranged in two hermetically sealed, moisture-resistant pouches, with 10 patches each.
To fix the patches in the correct position, 20 dermocompatible adhesive strips are included. A file is also provided to remove any remaining material from the surface of the wart.
Marketing authorization holder
Laboratorios Viñas, S.A.
Provenza, 386
08025 - Barcelona
Spain
Manufacturer
Laboratorios Viñas, S.A.
Torrente Vidalet, 29
08012- Barcelona
Spain
Date of the last revision of this leaflet:November 2024
Detailed information about this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.es/
- Country of registration
- Prescription requiredNo
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to VERRUPATCH 3.75 mg CUTANEOUS PATCHESDosage form: INJECTABLE, 1 mlActive substance: tralokinumabManufacturer: Leo Pharma A/SPrescription requiredDosage form: INJECTABLE, 300 mgActive substance: tralokinumabManufacturer: Leo Pharma A/SPrescription requiredDosage form: CAPSULE, 10 mgActive substance: alitretinoinManufacturer: Industrial Farmaceutica Cantabria S.A.Prescription required
Online doctors for VERRUPATCH 3.75 mg CUTANEOUS PATCHES
Discuss questions about VERRUPATCH 3.75 mg CUTANEOUS PATCHES, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions