
VENTOLIN 0.5 mg/ml INJECTABLE SOLUTION

How to use VENTOLIN 0.5 mg/ml INJECTABLE SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
- Introduction
- What is Ventolin 0.5 mg/ml Solution for Injection and what is it used for
- What you need to know before you use Ventolin 0.5 mg/ml Solution for Injection
- How to use Ventolin 0.5 mg/ml Solution for Injection
- Possible Side Effects
- Storage of Ventolin 0.5 mg/ml Solution for Injection
- Contents of the Pack and Further Information
Introduction
Package Leaflet: Information for the Patient
Ventolin 0.5 mg/ml Solution for Injection
salbutamol
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the Package Leaflet
- What is Ventolin 0.5 mg/ml Solution for Injection and what is it used for
- What you need to know before you use Ventolin 0.5 mg/ml Solution for Injection
- How to use Ventolin 0.5 mg/ml Solution for Injection
- Possible side effects
- Storage of Ventolin 0.5 mg/ml Solution for Injection
- Contents of the pack and further information
1. What is Ventolin 0.5 mg/ml Solution for Injection and what is it used for
Ventolin belongs to a group of medicines called bronchodilators. It relaxes the muscles of the walls of the small air passages in the lungs. This helps to open up the air passages and makes it easier to breathe, relieving wheezing, coughing, chest tightness, and shortness of breath.
Ventolin is indicated for the relief of severe bronchospasm associated with asthma or chronic bronchitis and for the treatment of status asthmaticus.
Ventolin injection is indicated for adults and adolescents.
2. What you need to know before you use Ventolin 0.5 mg/ml Solution for Injection
Do not use Ventolin 0.5 mg/ml Solution for Injection
- if you are allergic to salbutamol or any of the other ingredients of this medicine (listed in section 6).
- Non-intravenous preparations of Ventolin should not be used to stop premature labor or threatened abortion.
Warnings and Precautions
Consult your doctor or pharmacist before starting to use Ventolin if:
- you have had to stop using this or any other medicine for the treatment of this condition due to allergy or any other problem
- you have high blood pressure
- you have hyperthyroidism (increased activity of the thyroid gland)
- you have a history of heart problems such as rapid or irregular heartbeat or angina (chest pain)
- you have low levels of potassium in your blood
- you have diabetes mellitus (Ventolin may increase blood sugar levels)
- you have lactic acidosis (increased production of lactic acid)
- you are taking xanthine derivatives (such as theophylline) or steroids to treat asthma
- you are taking diuretics, which are sometimes used to treat high blood pressure or heart problems.
Your doctor will monitor your potassium levels if you are taking xanthine derivatives, steroids, or diuretics.
- Consult your doctor if you think you may have any of these problems.
Sometimes this medicine may not be suitable and your doctor may want to change it for another one.
Symptoms to be aware of
High doses of this medicine can occasionally produce a condition known as lactic acidosis. You should be aware of the appearance of certain symptoms while taking this medicine to reduce the risk of any problems. See "Symptoms to be aware of" in section 4.
Using Ventolin 0.5 mg/ml Solution for Injection with other medicines
Tell your doctor or pharmacist that you are taking, have recently taken, or might take any other medicines (e.g., treatments to eliminate fluids, any other type of bronchodilator tablets, steroids...), including those obtained without a prescription.
Also, inform your doctor if you are being treated with non-selective beta-blockers (mainly used for the treatment of heart rhythm disorders), such as propranolol, as these should not normally be administered with salbutamol.
Pregnancy and Breastfeeding
If you are pregnant or breastfeeding, or think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
If it is necessary to administer Ventolin during pregnancy or breastfeeding, your doctor will carefully weigh the benefits and potential risks based on the severity of the clinical condition.
Driving and Using Machines
No studies have been conducted on the ability to drive and use machines.
Important Information about Some of the Ingredients of Ventolin 0.5 mg/ml Solution for Injection
This medicine contains less than 23 mg (1 mmol) of sodium per ampoule; it is essentially "sodium-free".
Use in Athletes
This medicine contains salbutamol, which can produce a positive result in doping tests.
3. How to use Ventolin 0.5 mg/ml Solution for Injection
Follow exactly the administration instructions of this medicine indicated by your doctor. In case of doubt, consult your doctor or pharmacist again.
Ventolin injection is usually administered by a doctor or practitioner. It can be administered under the skin (subcutaneously), directly into a vein (intravenously), or into a muscle (intramuscularly).
The recommended dose administered subcutaneously or intramuscularly is 1 ampoule of 0.5 mg (500 micrograms).
The recommended dose administered intravenously is 0.25 mg (250 micrograms) injected very slowly.
Your doctor will indicate the duration of your treatment with Ventolin. Do not stop treatment before.
If you use more Ventolin 0.5 mg/ml Solution for Injection than you should
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone 91 562 04 20, indicating the medicine and the amount ingested.
At high doses as well as in overdose, an increase in lactic acid levels in the blood has been identified, which can cause difficulty breathing and hyperventilation.
If you forget to use Ventolin 0.5 mg/ml Solution for Injection
Do not use a double dose to make up for forgotten doses.
4. Possible Side Effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Symptoms to be aware of
The following adverse reactions are very rare (may affect less than 1 in 10,000 patients) but very serious. Contact your doctor immediatelyif you experience any of these symptoms. Stop using Ventolin.
Allergic Reactions:are very rare in patients using Ventolin. They include:
- sudden onset of wheezing or chest tightness
- swelling of eyelids, face, or lips
- skin rash (hives) or urticaria anywhere on the body
- sudden feeling of weakness or dizziness (may lead to collapse or loss of consciousness).
Lactic Acidosis: a very rare side effect of Ventolin administered intravenously is the increase in lactic acid in the blood (lactic acidosis). This affects more often patients with severe kidney problems. The symptoms of lactic acidosis include:
- rapid breathing, difficulty breathing
- feeling cold
- stomach pain, nausea, and vomiting.
The following side effects are associated with salbutamol, classified by organ system, frequency, and system.
The meaning of the terms used to describe the frequency of side effects is as follows: very common (may affect more than 1 in 10 patients), common (may affect up to 1 in 10 patients), uncommon (may affect up to 1 in 100 patients), rare (may affect up to 1 in 1,000 patients), and very rare side effects (may affect up to 1 in 10,000 patients).
Tell your doctor if you have any of the following symptoms:
Immune System Disorders
Very rare: hypersensitivity reactions including angioedema (skin reactions with redness, swelling, and itching), urticaria, bronchospasm (narrowing of the bronchial walls with decreased air intake), hypotension, and collapse.
Metabolism and Nutrition Disorders
Rare: hypokalemia (low potassium levels in the blood).
Therapy with beta-2 agonists, such as salbutamol, may lead to potentially serious hypokalemia.
Very rare: lactic acidosis.
Very rare cases of lactic acidosis have been reported in patients receiving salbutamol intravenously or by nebulization for the treatment of acute asthma exacerbations.
Nervous System Disorders
Very common: tremor.
Common: headache.
Very rare: hyperactivity.
Cardiac Disorders
Very common: tachycardia (increased heart rate), palpitations.
Rare: cardiac arrhythmias, including atrial fibrillation, supraventricular tachycardia, and extrasystoles (heart rhythm disorders).
Although the exact frequency is unknown, some people may occasionally experience chest pain (due to heart problems such as angina). Tell your doctor if you develop these symptoms while being treated with salbutamol, but do not stop taking it unless your doctor tells you to.
Vascular Disorders
Rare: peripheral vasodilation (dilation of peripheral blood vessels).
Respiratory, Thoracic, and Mediastinal Disorders
Uncommon: pulmonary edema (fluid accumulation in the lungs).
Gastrointestinal Disorders
Very rare: nausea, vomiting.
Musculoskeletal and Connective Tissue Disorders
Common: muscle cramps.
Injury, Poisoning, and Procedural Complications
Very rare: mild pain or stinging sensation in the case of intramuscular administration of the undiluted solution.
Reporting of Side Effects:
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Pharmacovigilance System for Human Use: www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Ventolin 0.5 mg/ml Solution for Injection
Keep this medicine out of the sight and reach of children.
Do not store above 30°C.
Store in the outer carton to protect from light.
Do not use this medicine after the expiry date which is stated on the carton after EXP. The expiry date is the last day of the month shown.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Contents of the Pack and Further Information
Composition of Ventolin 0.5 mg/ml Solution for Injection
- The active substance is salbutamol (as salbutamol sulfate). Each 1 ml ampoule contains 0.5 mg (500 micrograms) of salbutamol.
- The other ingredients are sodium chloride, sulfuric acid or sodium hydroxide (for pH adjustment), and water for injections.
Appearance of the Product and Contents of the Pack
Ventolin injection is a sterile normal saline solution. Each pack contains 5 or 6 ampoules of 1 ml.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder:
GlaxoSmithKline, S.A.
P.T.M. C/ Severo Ochoa, 2
28760 Tres Cantos (Madrid)
Tel: +34 900 202 700
Manufacturer:
GlaxoSmithKline Manufacturing S.p.A.
Strada Provinciale Asolana, 90 -43056 San Polo di Torrile (Parma) Italy
Date of Last Revision of this Leaflet: October 2024
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
-------------------------------------------------------------------------------------------------------------------------
This information is intended only for healthcare professionals
Dosage
Adults
Subcutaneous and intramuscular route: the normal dose is 1 ampoule with 0.5 mg (500 micrograms) at a rate of 8 micrograms/kg. The dose can be repeated every 4 hours if necessary.
Intravenous route: the normal dose is 0.25 mg (250 micrograms) at a rate of 4 micrograms/kg injected very slowly. If necessary, the dose can be repeated.
Use in Children
Safety and efficacy have not been established in children under 12 years of age.
In children 12 years and older: use the same dose as in the adult population.
Method of Administration
Ventolin injection can be diluted to facilitate administration. The only suitable solvents are: water for injections, sodium chloride injection solution, glucose injection solution, or sodium chloride and glucose injection solution.
Any unused mixtures of Ventolin 0.5 mg/ml Solution for Injection for fluid perfusion should be discarded 24 hours after preparation.
Whenever possible, oxygen administration is recommended in conjunction with Ventolin injection, mainly in hypoxic patients.
Do not mix in the same syringe with other medicines.
Instructions for Opening the Ventolin Injection Ampoule:
The ampoules have a "One Point Cut" (UPC) opening system and should be opened following the instructions below:
- Hold the lower part of the ampoule with one hand, as indicated in Figure 1.
- Place the other hand on the upper part of the ampoule, positioning the thumb on the colored point and pressing, as indicated in Figure 2.
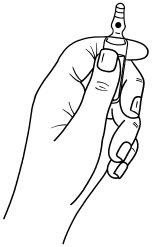
Figure 1
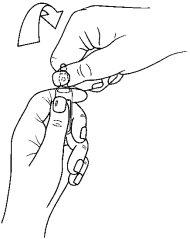
Figure 2
The detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Average pharmacy price2.19 EUR
- Availability in pharmacies
Supply issue reported
Data from the Spanish Agency of Medicines (AEMPS) indicates a supply issue affecting this medicine.<br><br>Availability may be limited in some pharmacies.<br><br>For updates or alternatives, consult your pharmacist. - Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to VENTOLIN 0.5 mg/ml INJECTABLE SOLUTIONDosage form: ORAL SOLUTION/SUSPENSION, 0.0482 gActive substance: salbutamolManufacturer: Laboratorio Aldo Union S.L.Prescription requiredDosage form: ORAL SOLUTION/SUSPENSION, 2 mg salbutamol sulfate / 5 mlActive substance: salbutamolManufacturer: Glaxosmithkline S.A.Prescription requiredDosage form: ORAL SOLUTION/SUSPENSION, 0.03 gActive substance: terbutalineManufacturer: Laboratorios Ern S.A.Prescription required
Online doctors for VENTOLIN 0.5 mg/ml INJECTABLE SOLUTION
Discuss questions about VENTOLIN 0.5 mg/ml INJECTABLE SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















