
Salbutamol Vzf
Ask a doctor about a prescription for Salbutamol Vzf

How to use Salbutamol Vzf
Leaflet attached to the packaging: patient information
SALBUTAMOL WZF, 0.5 mg/ml, solution for injection
Salbutamol
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, so you can read it again if you need to.
- If you have any doubts, consult your doctor, pharmacist, or nurse.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor, pharmacist, or nurse. See section 4.
Table of contents of the leaflet
- 1. What is Salbutamol WZF and what is it used for
- 2. Important information before using Salbutamol WZF
- 3. How to use Salbutamol WZF
- 4. Possible side effects
- 5. How to store Salbutamol WZF
- 6. Contents of the packaging and other information
1. What is Salbutamol WZF and what is it used for
Salbutamol WZF belongs to a group of medicines called beta-adrenergic agonists. It causes relaxation of the bronchial muscles and airways.
Salbutamol WZF in the form of a solution for injection is used:
- in severe bronchospastic conditions (e.g., severe breathing difficulties in patients with asthma).
2. Important information before using Salbutamol WZF
When not to use Salbutamol WZF
- If the patient is allergic to salbutamol sulfate or any of the other ingredients of this medicine (listed in section 6).
- If the patient is pregnant for less than 22 weeks.
- If the patient has ischemic heart disease or a risk of developing ischemic heart disease (a disease characterized by reduced blood flow to the heart muscle, which can cause symptoms such as chest pain).
- If the patient has had a miscarriage during the first two trimesters of pregnancy.
- If the patient is pregnant and has certain diseases or disorders that make it dangerous to prolong pregnancy (such as severe hypertension, intrauterine infection, bleeding, placenta previa, or intrauterine fetal death).
- If the patient has heart disease with arrhythmia (e.g., heart valve defect) or chronic lung disease (e.g., chronic bronchitis, pulmonary emphysema), which can cause increased blood pressure in the pulmonary vessels (pulmonary hypertension).
Warnings and precautions
Before starting treatment with Salbutamol WZF, discuss it with your doctor or nurse.
- If the patient has the following health problems, they should tell their doctor before using the medicine:
- heart and blood circulation disorders (symptoms such as chest pain, rapid or irregular heartbeat, shortness of breath, high blood pressure, rapid pulse), see section "When not to use Salbutamol WZF",
- hyperthyroidism,
- epilepsy,
- diabetes (high blood sugar levels).
- Caution should be exercised when Salbutamol WZF is used as the only or primary medicine in patients with severe or unstable asthma, as there is a risk of a severe asthma attack.
- If the patient experiences symptoms of an allergic reaction, such as bronchospasm, throat swelling, or skin rash, especially in children, they should contact their doctor immediately - see section 4 "Possible side effects".
- During treatment, the patient should not increase the dose or frequency of administration without consulting their doctor.
Salbutamol WZF and other medicines
Tell your doctor about all medicines you are currently taking or have recently taken, as well as any medicines you plan to take.
- Salbutamol WZF should not be used at the same time as other bronchodilator medicines taken orally or intravenously, as this may cause severe side effects on the cardiovascular system, such as rapid or irregular heartbeat.
- Salbutamol WZF can only be used after consulting a doctor:
- with medicines used for irregular or rapid heart rhythm (such as digoxin);
- with beta-adrenergic receptor blockers (such as atenolol or propranolol), including eye drops (e.g., timolol), as this may cause bronchospasm in patients with asthma;
- with xanthine derivatives (e.g., theophylline or aminophylline);
- with diuretics, especially those that cause potassium loss, e.g., furosemide;
- with steroid medicines (e.g., prednisolone);
- with medicines used to treat diabetes to reduce blood sugar levels (e.g., insulin, metformin, or glibenclamide);
- with antidepressant medicines from the group of monoamine oxidase inhibitors and tricyclic antidepressants.
In the event of a planned surgical procedure with the use of general anesthesia, the doctor will discontinue the administration of Salbutamol WZF 6 hours before the procedure, if possible, to protect the patient from side effects (e.g., irregular heartbeat).
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult their doctor before using this medicine.
The medicine can inhibit uterine contractions and may make delivery difficult.
The doctor may recommend the use of Salbutamol WZF during pregnancy only in cases where the benefit to the mother outweighs the potential risk to the fetus.
Salbutamol is likely to pass into breast milk. If the use of the medicine is necessary, the patient should stop breastfeeding.
Driving and operating machinery
There is no data on the effect of salbutamol on driving and operating machinery.
Salbutamol WZF contains sodium
Salbutamol WZF contains 3.55 mg of sodium (the main component of table salt) per ml of solution.
This corresponds to 0.18% of the maximum recommended daily intake of sodium in the diet for adults.
The medicine can be diluted in a 0.9% NaCl solution or a 5% glucose solution. The sodium content from the diluent should be taken into account when calculating the total sodium content in the prepared dilution of the medicine. For accurate information on the sodium content in the solution used to dilute the medicine, refer to the patient information leaflet for the diluent.
3. How to use Salbutamol WZF
- Salbutamol WZF is administered by medical personnel.
- The medicine can be administered subcutaneously, intramuscularly, or intravenously.
- The dosage is determined individually by the doctor for each patient.
Adults
Subcutaneously or intramuscularly: 0.5 mg (8 micrograms/kg body weight). If necessary, the dose can be repeated every 4 hours.
Intravenously: inject slowly from 0.25 mg to 0.5 mg (from 4 micrograms/kg body weight to 8 micrograms/kg body weight).
If necessary, the dose of the medicine can be repeated after 15 minutes, preferably in a drip infusion at a rate of 5 micrograms/min, monitoring the pulse and blood pressure.
Note:during intravenous administration, oxygen therapy and potassium supplementation are recommended. The medicine can also be used by nebulization in a dose of 2.5 mg to 5 mg (in asthmatic conditions every 1 to 3 hours).
Children
There is insufficient data to determine the dosage of salbutamol in the form of a solution for injection in children.
Detailed information on dosage is provided in the section "Information intended exclusively for healthcare professionals".
Using a higher dose of the medicine than recommended
After using a higher dose than recommended, the following may occur: chest pain, increased or decreased blood pressure, rapid or irregular heartbeat, nervousness, headache, tremors, convulsions, dryness of the mucous membranes, dizziness, nausea, insomnia. A decrease in potassium levels in the blood may occur.
If a higher dose of the medicine than recommended is used, the patient should immediately consult their doctor, who will provide appropriate treatment.
Missing a dose of Salbutamol WZF
The medicine is administered by medical personnel, so missing a dose is unlikely.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
If the patient experiences the first symptoms of hypersensitivity (e.g., facial swelling, lip swelling, tongue swelling, throat swelling, causing difficulty breathing or swallowing, bronchospasm, acute cardiovascular failure), they should contact their doctor immediately. Such symptoms are very rare.
Very common (more than 1 in 10 people):
- tremors;
- rapid heartbeat.
Common (less than 1 in 10 people):
- decreased potassium levels in the blood, which can cause muscle weakness, increased thirst, or a tingling sensation;
- headache;
- palpitations (rapid heartbeat);
- decreased blood pressure, which can cause dizziness or lightheadedness;
- muscle cramps.
Uncommon (less than 1 in 100 people):
- fluid accumulation in the lungs (pulmonary edema), which can cause breathing difficulties.
Rare (less than 1 in 1,000 people):
- increased blood sugar and/or lactic acid levels;
- heart rhythm disorders (including atrial fibrillation, supraventricular tachycardia, and ventricular extrasystoles), myocardial ischemia;
- abnormal or irregular heartbeat;
- sudden flushing of the face;
- peripheral vasodilation.
Very rare (less than 1 in 10,000 people):
- hyperactivity;
- muscle stiffness;
- hives.
Very rarely, some people may experience chest pain (related to heart diseases such as angina). If such symptoms occur, the patient should not suddenly stop taking the medicine but should contact their doctor as soon as possible.
Reporting side effects
If side effects occur, including any side effects not listed in this leaflet, the patient should tell their doctor, pharmacist, or nurse. Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products
Al. Jerozolimskie 181C
02-222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help gather more information on the safety of the medicine.
5. How to store Salbutamol WZF
Store in a temperature below 25°C.
Store the ampoules in the outer packaging to protect them from light, do not freeze.
The medicine should be stored out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the box and ampoule. The expiry date refers to the last day of the month.
The inscription on the packaging after the abbreviation EXP indicates the expiry date, and after the abbreviation Lot indicates the batch number.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Salbutamol WZF contains
- The active substance of the medicine is salbutamol sulfate. Each ml of solution contains 0.5 mg of salbutamol (in the form of salbutamol sulfate).
- The other ingredients are: disodium edetate, sodium chloride, 10% hydrochloric acid (to adjust pH), water for injections.
What Salbutamol WZF looks like and what the packaging contains
Salbutamol WZF is a colorless, clear liquid.
The packaging contains 10 glass ampoules with a capacity of 1 ml in a cardboard box.
Marketing authorization holder and manufacturer
Zakłady Farmaceutyczne POLPHARMA S.A.
ul. Pelplińska 19, 83-200 Starogard Gdański
phone: +48 22 364 61 01
Date of the last update of the leaflet:
Information intended exclusively for healthcare professionals:
SALBUTAMOL WZF, 0.5 mg/ml, solution for injection
Salbutamol
- The dosage is determined individually by the doctor for each patient.
- The medicine is administered subcutaneously, intramuscularly, or intravenously. When administered intravenously, oxygen therapy and potassium supplementation are recommended.
- The medicine can be diluted in a 0.9% NaCl solution (see section 2, "Salbutamol WZF contains sodium") or a 5% glucose solution. After dilution, the resulting solution can be stored for no longer than 24 hours.
- In bronchospastic conditions, the medicine quickly (within 5 minutes) but briefly (4 to 6 hours) relaxes the airways.
Instructions for opening the ampoule
Before opening the ampoule, make sure the entire solution is in the lower part of the ampoule.
You can gently shake the ampoule or tap it with your finger to facilitate the flow of the solution.
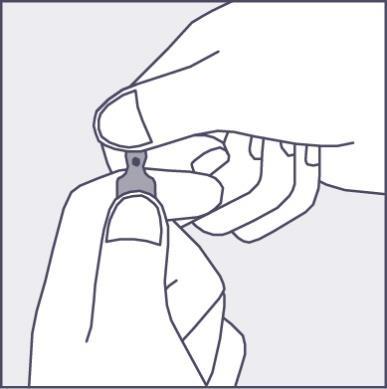
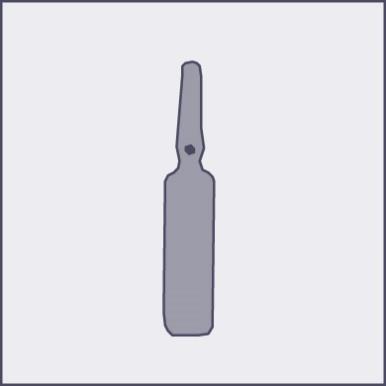
A colored dot is placed on each ampoule (see Figure 1) as a mark indicating the location of the break point below it.
- To open the ampoule, hold it vertically, with both hands, with the colored dot facing you - see Figure 2. The upper part of the ampoule should be grasped in such a way that the thumb is above the colored dot.
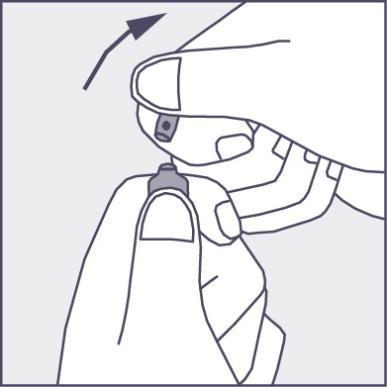
- Press according to the arrow on Figure 3. The ampoules are intended for single use only and should be opened immediately before use. The remaining contents of the unused product should be destroyed in accordance with applicable regulations.
Figure 1
Figure 2
Figure 3
Dosage
Adults
Subcutaneously or intramuscularly: 0.5 mg (8 micrograms/kg body weight). If necessary, the dose can be repeated every 4 hours.
Intravenously: inject slowly from 0.25 mg to 0.5 mg (from 4 micrograms/kg body weight to 8 micrograms/kg body weight).
Before administration, 0.5 mg of salbutamol (1 ml of solution) can be diluted to 10 ml with water for injections (concentration after dilution 50 micrograms/ml) and administered slowly intravenously at 5 ml of the diluted medicine (250 micrograms/5 ml).
If necessary, the dose of the medicine can be repeated after 15 minutes, preferably in a drip infusion at a rate of 5 micrograms/min, monitoring the pulse and blood pressure.
Note:during intravenous administration, oxygen therapy and potassium supplementation are recommended. The medicine can also be used by nebulization in a dose of 2.5 mg to 5 mg (in asthmatic conditions every 1 to 3 hours).
In severe cases of asthma during the administration of salbutamol, it is necessary to determine the potassium level in the serum, as it may decrease significantly (especially if other medicines have been used, e.g., methylxanthines, steroids, diuretics, laxatives).
Children
There is insufficient data to determine the dosage of salbutamol in the form of a solution for injection in children.
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterZakłady Farmaceutyczne POLPHARMA S.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Salbutamol VzfDosage form: Syrup, 2 mg/5 mlActive substance: salbutamolPrescription requiredDosage form: Tablets, 2 mgActive substance: salbutamolPrescription requiredDosage form: Tablets, 4 mgActive substance: salbutamolPrescription required
Alternatives to Salbutamol Vzf in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Salbutamol Vzf in Spain
Alternative to Salbutamol Vzf in Ukraine
Online doctors for Salbutamol Vzf
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Salbutamol Vzf – subject to medical assessment and local rules.














