VAXCHORA effervescent powder and powder for oral suspension

How to use VAXCHORA effervescent powder and powder for oral suspension
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Vaxchora Effervescent Powder and Powder for Oral Suspension
Cholera Vaccine (Recombinant, Live, Oral)
Read all of this leaflet carefully before taking this vaccine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This vaccine has been prescribed for you, do not pass it on to others.
- If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. See section 4.
Contents of the Package Leaflet
- What is Vaxchora and what is it used for
- What you need to know before taking Vaxchora
- How to take Vaxchora
- Possible side effects
- Storage of Vaxchora
- Contents of the pack and other information
1. What is Vaxchora and what is it used for
Vaxchora is an oral vaccine against cholera that stimulates the immune defense in the intestine. The vaccine is used for protection against cholera in adults and children from 2 years of age. The vaccine should be taken at least 10 days before traveling to a cholera-affected area.
How Vaxchora works
Vaxchora prepares the immune system (the body's defenses) to defend against cholera. When the person takes the vaccine, the immune system produces proteins called antibodies against the cholera bacteria and its toxin, a harmful substance that causes diarrhea. In this way, the immune system is prepared to fight against the cholera bacteria if the person comes into contact with them.
2. What you need to know before taking Vaxchora
Do not take Vaxchora:
- if you are allergic to any of the components of this medicine (listed in section 6).
- if your immune system is weakened, either from birth or because you are receiving treatments that weaken it, such as high doses of corticosteroids, cancer medicines, or radiotherapy.
Warnings and precautions
Talk to your doctor, pharmacist, or nurse before taking Vaxchora.
Contact a doctor immediately if you experience the following serious side effects (see also section 4):
- severe allergic reactions that cause swelling in the face or throat, hives, itchy rash, and shortness of breath and/or a drop in blood pressure and fainting.
If you experience any of the following effects or a combination of nausea, vomiting, diarrhea, or abdominal pain, talk to your doctor, pharmacist, or nurse before taking the vaccine. Vaccination should be postponed until after recovery, as protection against cholera may be reduced.
Not everyone who takes this vaccine will be completely protected against cholera. It is essential to follow hygiene advice and take special care with food, diaper changes, and water consumed in cholera-affected areas.
This vaccine may be less effective if you have HIV.
The bacteria in the vaccine may be present in the stool for at least 7 days after taking the vaccine. To avoid contamination, wash your hands well after using the bathroom and before preparing food for at least 14 days after taking this vaccine.
Children and adolescents
Do not give this vaccine to children under 2 years of age, as it is not known if it works well in this age group.
Other medicines and Vaxchora
Tell your doctor, pharmacist, or nurse if you are taking, have recently taken, or might take any other medicine or vaccine. This includes medicines obtained without a prescription, including herbal remedies. This is because this vaccine may affect how other medicines or vaccines work.
In particular, talk to your doctor, pharmacist, or nurse if you are taking:
- Antibiotics: this vaccine may not work if taken at the same time as antibiotics. Do not take this vaccine before 14 days after the last dose of an antibiotic. Avoid antibiotics for at least 10 days after taking this vaccine.
- Chloroquine for malaria prevention: this vaccine may not work if taken at the same time as chloroquine. Take this vaccine at least 10 days before starting chloroquine or 14 days after chloroquine treatment.
- Ty21a typhoid vaccine: this vaccine may not work if taken at the same time as Ty21a. You should take this vaccine at least 2 hours before or after Ty21a.
If any of the above applies to you, talk to your doctor, pharmacist, or nurse before taking this vaccine.
Taking Vaxchora with food and drinks
You should not eat or drink for 60 minutes before and after taking this vaccine, as this may interfere with the vaccine's effectiveness.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
Driving and using machines
Vaxchora vaccine has no or negligible influence on the ability to drive and use machines. However, some of the effects mentioned in section 4 "Possible side effects" may temporarily affect your ability to drive or use machines. Do not drive or use machines if you do not feel well after vaccination.
Vaxchora contains lactose, sucrose, and sodium
If your doctor has told you that you have an intolerance to some sugars, talk to them before taking this medicine.
This vaccine contains 863 mg of sodium (the main component of table salt/cooking salt) per dose. This is equivalent to 43% of the recommended daily sodium intake for an adult. Keep this in mind if you are on a low-sodium diet.
3. How to take Vaxchora
Follow the instructions for administering this vaccine exactly as indicated by your doctor, pharmacist, or nurse. If in doubt, consult your doctor, pharmacist, or nurse again.
The recommended dose is the contents of the two sachets in the box. However, for children between 2 and less than 6 years of age, note the Step 8 instructions for preparing the vaccine, shown below.
Protection against cholera is established 10 days after taking Vaxchora. Your doctor, pharmacist, or nurse will tell you how long before traveling you should take the vaccine.
Instructions:
FOLLOW THE PREPARATION INSTRUCTIONS FOR THIS VACCINE CONTAINED IN THIS LEAFLET Read the following before starting: Vaxchora may not work if the following occurs:
Do not touch your eyes during vaccine preparation to avoid contamination. If a spill occurs, clean the surface with hot water and soap or an antibacterial disinfectant. If a significant spill occurs (more than a few drops of liquid or grains of powder), discard the vaccine and request a new one from your doctor or pharmacist. DO NOT take the remaining medicine. | |
Step 1 | Gather the materials:
|
Step 2
| Remove the vaccine from the refrigerator. |
Step 3
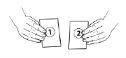
| Locate the two sachets: the sachets are labeled as 1 and 2. Sachet 1 contains the "Vaxchora buffer" and is black and white in color. Sachet 2 contains the "Vaxchora active ingredient" and is blue and white in color. If a sachet is not intact, do not use either sachet and contact your doctor, pharmacist, or nurse to obtain a replacement dose. If you use a sachet that is not intact, the vaccine's effectiveness may be reduced. |
Step 4
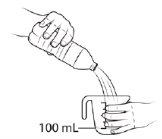
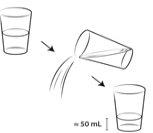
| Measure 100 ml of bottled water with or without gas, cold or at room temperature, and pour it into a clean glass. The use of bottled water is necessary for the vaccine to be effective; if you use non-bottled water (e.g., tap water), the vaccine may be ineffective. |
Step 5
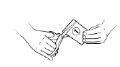
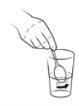
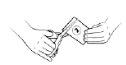
| Use the scissors to cut the top of sachet 1. Do not put your fingers inside the sachet. Wash your hands if you touch the contents of the sachet to reduce the possibility of contamination. |
Step 6
| Empty the contents of sachet 1 into the water in the glass. It is effervescent. |
Step 7
| Mix until the powder is completely dissolved. |
Step 8 | Only for children between 2 and less than 6 years of age: Shake and discard half of the buffer solution. (Note: For children over 6 years and adults, THIS STEP IS NOT REQUIRED). |
Step 9
| Use the scissors to cut the top of sachet 2. Do not put your fingers inside the sachet. Wash your hands if you touch the contents of the sachet to reduce the possibility of contamination. |
Step 10
| Empty the contents of sachet 2 into the glass. |
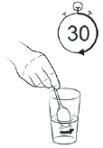
Step 11
| Mix for at least 30 seconds. The powder from sachet 2 may not dissolve completely. It will form a slightly cloudy mixture with some white particles. If desired, after shaking sachet 2 for at least 30 seconds, you can add a sweetener with stevia (no more than 1 gram or ¼ teaspoon) or sugar (sucrose, no more than 4 grams or 1 teaspoon) and then shake in the suspension. DO NOT add other sweeteners, as this may reduce the effectiveness of the vaccine. |
Step 12
| Drink the entire contents of the glass within 15 minutes of preparation. Some residue may remain in the glass, which should be discarded. If you or your child take less than half of the dose, contact your doctor, pharmacist, or nurse immediately if a repeat dose is needed. |
Step 13
| Discard the empty sachets according to local biosecurity guidelines. Ask your doctor, pharmacist, or nurse how to dispose of the packaging and any materials that have come into contact with the medicine. |
Step 14
| If a spill occurs while mixing or taking the medicine, or if any residue (powder or liquid on the stirring utensil, glass, or other object) remains on the mixing surface, clean the spilled material or residue preferably with hot water and soap, or an antibacterial disinfectant, and a cloth or disposable paper towel. Discard the paper towel along with the sachets (see above). |
Step 15
| Wash the glass and spoon or stirrer with soap and hot water. |
Step 16
| Wash your hands well with soap and hot water to avoid contamination. |
If you have any further questions on the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all vaccines, this vaccine can cause side effects, although not everybody gets them.
Contact a doctor immediately if you experience the following serious side effects:
- severe allergic reactions that cause swelling in the face or throat, hives, itchy rash, and shortness of breath or a drop in blood pressure and fainting.
Other side effects:
Very common side effects (may affect more than 1 in 10 people):
- fatigue,
- headache,
- stomach pain,
- feeling dizzy or vomiting,
- decreased appetite,
Common side effects (may affect up to 1 in 10 people):
- diarrhea.
Uncommon side effects (may affect up to 1 in 100 people):
- fever,
- joint pain,
- dizziness,
- gas (abdominal distension),
- rash,
- dry mouth,
- constipation,
- flatulence,
- belching,
- indigestion,
- abnormal stools
Rare side effects (may affect up to 1 in 1,000 people):
- chills.
Additional side effects in children and adolescents
According to clinical trials, it is expected that adverse reactions in children will be of a similar type to those in adults. Some adverse reactions were more frequent in children than in adults, such as fatigue, stomach pain, vomiting, decreased appetite, and fever.
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Vaxchora
Keep this vaccine out of the sight and reach of children.
Do not use this vaccine after the expiry date that appears on the carton. The expiry date is the last day of the month indicated.
Store in a refrigerator (between 2°C and 8°C).
Store in the original packaging to protect it from light and moisture.
In its original packaging, the vaccine is stable for up to 12 hours at 25°C. At the end of this period, the vaccine must be used immediately or discarded. Avoid exposing Vaxchora to temperatures above 25°C.
Do not use this vaccine if you notice that the sachets are damaged and contact your doctor, pharmacist, or nurse to obtain a replacement dose.
This medicine contains genetically modified organisms. Local biosecurity guidelines should be followed for the disposal of unused medicine and all materials that have come into contact with it. Ask your doctor, pharmacist, or nurse how to dispose of the packaging and any materials that have come into contact with the medicine.
6. Contents of the pack and other information
Composition of Vaxchora
- Each dose contains 4 × 10^8 to 2 × 10^9 viable cells of the CVD 103-HgR strain of Vibrio Cholerae1.
- The other components are sucrose, hydrolyzed casein, ascorbic acid, lactose, sodium bicarbonate, and sodium carbonate (see section 2. Vaxchora contains lactose, sucrose, and sodium).
- This vaccine contains genetically modified organisms (GMOs).
1 Produced using recombinant DNA technology.
Appearance and packaging of the product
A dose of Vaxchora effervescent powder and powder for oral suspension is presented in a carton containing two sachets. One sachet contains the white to off-white effervescent powder buffer. The other sachet contains the white to beige active ingredient powder for oral suspension.
Marketing authorization holder
Bavarian Nordic A/S, Philip Heymans Alle 3, DK-2900 Hellerup, Denmark
Manufacturer
IL-CSM GmbH
Marie-Curie-Strasse 8
D-79539 Lörrach
Germany
Date of last revision of this leaflet:01/2025.
Other sources of information
Detailed information on this medicine is available on the European Medicines Agency website: https://www.ema.europa.eu.
- Country of registration
- Average pharmacy price49.39 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to VAXCHORA effervescent powder and powder for oral suspensionDosage form: ORAL SOLUTION/SUSPENSION, 100 BILLION / 1 mgActive substance: cholera, inactivated, whole cellManufacturer: Valneva Sweden AbPrescription requiredDosage form: INJECTABLE, 0.5 ml single doseActive substance: meningococcus B, multicomponent vaccineManufacturer: Glaxosmithkline Vaccines S.R.L.Prescription requiredDosage form: INJECTABLE, 0.5 ml single doseActive substance: meningococcus B, multicomponent vaccineManufacturer: Glaxosmithkline Vaccines S.R.L.Prescription required
Online doctors for VAXCHORA effervescent powder and powder for oral suspension
Discuss questions about VAXCHORA effervescent powder and powder for oral suspension, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions