VABYSMO 120 mg/mL solution for injection in pre-filled syringe

How to use VABYSMO 120 mg/mL solution for injection in pre-filled syringe
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Vabysmo 120mg/ml solution for injection in a pre-filled syringe
faricimab
This medicine is subject to additional monitoring, which will allow for quick identification of new safety information. You can help by reporting any side effects you may get. See the end of section 4 for how to report side effects.
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor.
- If you get any side effects, talk to your doctor, even if you think they might be unrelated to the medicine. See section 4.
Contents of the pack
- What is Vabysmo and what is it used for
- What you need to know before you use Vabysmo
- How to use Vabysmo
- Possible side effects
- Storage of Vabysmo
- Contents of the pack and other information
1. What is Vabysmo and what is it used for
What is Vabysmo and what is it used for
Vabysmo contains the active substance faricimab, which belongs to a group of medicines called anti-neovascularisation agents.
Vabysmo is injected into the eye by your doctor to treat eye disorders in adults called:
- neovascular (exudative) age-related macular degeneration (nAMD),
- vision impairment due to diabetic macular edema (DME).
- vision impairment due to macular edema resulting from blockage of the retinal veins (branch retinal vein occlusion (BRVO) or central retinal vein occlusion (CRVO)).
These disorders affect the macula, the central part of the retina (the light-sensitive layer at the back of the eye) that is responsible for fine and central vision. nAMD occurs due to the growth of abnormal blood vessels that allow blood and fluid to leak into the macula, and DME occurs due to leaky blood vessels causing swelling of the macula. CRVO is the blockage of the main blood vessel (vein) that carries blood from the retina, and BRVO is the blockage of one of the smaller branches of the main vein. Due to increased pressure within the veins, there is a leakage of fluid into the retina, causing macular swelling (edema).
How Vabysmo works
Vabysmo specifically recognizes and blocks the activity of certain proteins known as angiopoietin-2 and vascular endothelial growth factor A. When these proteins are present at higher-than-normal levels, they can cause the growth of abnormal blood vessels and/or damage to normal blood vessels, with leakage into the macula, causing swelling or damage that can negatively affect a person's vision. By binding to these proteins, Vabysmo can block their actions and prevent abnormal blood vessel growth, leakage, and swelling. Vabysmo may improve the disease and/or slow down the worsening of the disease, and thus maintain, or even improve, your vision.
2. What you need to know before you use Vabysmo
Do not use Vabysmo:
- if you are allergic to faricimab or any of the other ingredients of this medicine (listed in section 6).
- if you have an active infection or suspected infection in or around the eye.
- if you have pain or redness in the eye (ocular inflammation).
If you are in any of these situations, tell your doctor. You should not be given Vabysmo.
Warnings and precautions
Talk to your doctor before you start using Vabysmo:
- if you have glaucoma (an eye disease usually caused by high pressure in the eye).
- if you have a history of seeing flashes, lights, or floating particles (floaters) and if you have a sudden increase in the size and number of floaters.
- if you have had eye surgery in the last 4 weeks or if eye surgery is planned for the next 4 weeks.
- if you have ever had any other eye disease or eye treatment.
Talk to your doctor immediately if:
- you experience sudden loss of vision.
- you experience signs of a possible infection or ocular inflammation, such as worsening eye redness, eye pain, increased eye discomfort, blurred or decreased vision, an increase in the number of small particles in your vision, increased sensitivity to light.
Also, it is important that you know that:
- the safety and efficacy of Vabysmo when administered in both eyes at the same time have not been studied, and using it in this way may increase the risk of experiencing side effects.
- injections with Vabysmo may cause a temporary increase in eye pressure (intraocular pressure) in some patients within 60 minutes after the injection. Your doctor will monitor this after each injection.
- Your doctor will check if you have other risk factors that may increase the likelihood of a tear or detachment of one of the layers of the back of the eye (retinal detachment or tear, and retinal pigment epithelial tear or detachment), in which case Vabysmo will be administered with caution.
When some medicines that work in a similar way to Vabysmo are administered, it is known that there is a risk of blood clots that block blood vessels (arterial thromboembolic events), which can cause a heart attack or stroke. Since a small amount of the medicine enters the bloodstream, there is a theoretical risk of these episodes after injection of Vabysmo into the eye.
Experience is limited in the treatment of:
- patients with active infections.
- patients with nAMD and patients with retinal vein occlusion (RVO) 85 years and older.
- patients with DME due to type 1 diabetes.
- diabetics with high average blood sugar levels (HbA1c above 10%).
- diabetics with eye disease caused by diabetes, known as proliferative diabetic retinopathy.
- diabetics with high blood pressure above 140/90 mmHg and vascular disease.
- patients with DME who receive injections at intervals of less than 8 weeks over a long period.
Experience is limited in the treatment of patients who receive injections at intervals of less than 8 weeks over a long period, and these patients may have a higher risk of side effects.
There is no experience in the treatment of:
- diabetics or patients with RVO with uncontrolled high blood pressure.
If any of the above applies to you, your doctor will take into account this lack of information when treating you with Vabysmo.
Children and adolescents
The use of Vabysmo has not been studied in children and adolescents because nAMD, DME, and RVO mainly occur in adults.
Other medicines and Vabysmo
Tell your doctor if you are using, have recently used, or might use any other medicines.
Pregnancy andbreast-feeding
Vabysmo has not been studied in pregnant women. Vabysmo should not be used during pregnancy unless the potential benefit to the patient outweighs the potential risk to the unborn child.
If you are pregnant or breast-feeding, think you may be pregnant, or are planning to have a baby, ask your doctor for advice before using this medicine.
Breast-feeding is not recommended during treatment with Vabysmo because it is not known whether Vabysmo is excreted in human milk.
Women who could become pregnant should use an effective method of contraception during treatment and for at least 3 months after the end of treatment with Vabysmo. If you become pregnant or think you may be pregnant during treatment, tell your doctor immediately.
Driving and using machines
After injection of Vabysmo, you may experience temporary vision problems (e.g., blurred vision). Do not drive or use machines while these last.
Vabysmo contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per dose, which is essentially "sodium-free".
Vabysmo contains polysorbate
This medicine contains 0.02 mg of polysorbate in each 0.05 ml dose. Polysorbates may cause allergic reactions. Talk to your doctor if you have any known allergies.
3. How to use Vabysmo
How Vabysmo is administered
The recommended dose is 6 mg of faricimab.
Neovascular (exudative) age-related macular degeneration (nAMD)
- You will be given an injection every month for the first 3 months.
- After that, you will receive injections every 4 months. Your doctor will determine the frequency of injections based on the condition of your eye.
Diabetic macular edema (DME) and macular edema secondary to retinal vein occlusion (branch retinal vein occlusion (BRVO) or central retinal vein occlusion (CRVO))
- You will be given an injection every month for at least 3 months.
- After that, you may receive injections less frequently. Your doctor will determine the frequency of injections based on the condition of your eye.
Method of administration
Vabysmo is injected into the eye (intravitreal injection) by an experienced doctor in administering eye injections.
Before the injection, your doctor will use an eye disinfectant to carefully clean your eye to prevent infection. Your doctor will give you an eye drop (local anesthetic) to numb the eye and reduce or prevent pain from the injection.
How long the treatment with Vabysmo will last
This is a long-term treatment, which may continue for months or years. Your doctor will regularly monitor your condition to check that the treatment is having the desired effect. Depending on how you respond to treatment with Vabysmo, your doctor may change the frequency of doses to more or less often.
If you miss a dose of Vabysmo
If you miss a dose, make a new appointment with your doctor as soon as possible.
If you stop treatment with Vabysmo
Talk to your doctor before stopping treatment. Stopping treatment may increase the risk of vision loss, and your vision may worsen.
If you have any other questions about the use of this medicine, ask your doctor.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Side effects with Vabysmo injections are both due to the medicine and the injection procedure and may mainly affect the eye.
Some side effects may be serious
Contact your doctor immediatelyif you have any of the following symptoms, which are signs of allergic reactions, inflammation, or infections:
- eye pain, increased discomfort, worsening eye redness, blurred or decreased vision, increase in the number of small particles in your vision, increased sensitivity to light – these are signs of a possible eye infection, inflammation, or allergic reaction.
- a sudden decrease or change in vision.
Other possible side effects
Other side effects that may occur after treatment with Vabysmo include those listed below.
Many of the side effects are mild or moderate and will usually go away within a week after each injection.
Contact your doctor if any of the following side effects become serious.
Very common(may affect more than 1 in 10 people):
- None
Common(may affect up to 1 in 10 people):
- Clouding of the lens in the eye (cataract)
- Tear of one of the layers of the back of the eye (retinal pigment epithelial tear, only in nAMD)
- Detachment of the gel-like substance inside the eye (vitreous detachment)
- Increased pressure inside the eye (increased intraocular pressure)
- Bleeding from the small blood vessels of the outer layer of the eye (conjunctival hemorrhage)
- Moving spots or dark shadows in your vision (vitreous floaters)
- Eye pain
Uncommon(may affect up to 1 in 100 people):
- Severe inflammation or infection inside the eye (endophthalmitis)
- Inflammation of the gel-like substance inside the eye/eye redness (vitritis)
- Inflammation of the iris and its surrounding tissue in the eye (iritis, iridocyclitis, uveitis)
- Bleeding inside the eye (vitreous hemorrhage)
- Eye discomfort
- Itching (ocular pruritus)
- Tear of the retina (back of the eye that detects light)
- Eye redness (ocular hyperemia/conjunctival)
- Feeling of having something in the eye
- Blurred vision
- Decreased sharpness of vision (reduced visual acuity)
- Pain during the procedure
- Retinal detachment
- Increased production of tears (increased lacrimation)
- Scratching of the cornea, damage to the transparent part of the eyeball that covers the iris (corneal abrasion)
- Ocular irritation
Rare(may affect up to 1 in 1,000 people):
- Temporary decrease in sharpness of vision (transiently reduced visual acuity)
- Clouding of the lens due to an injury (traumatic cataract)
Frequency not known
- Retinal vasculitis (inflammation of the blood vessels in the back of the eye)
- Occlusive retinal vasculitis (blockage of the blood vessels in the back of the eye, usually in the presence of inflammation)
When some medicines that work in a similar way to Vabysmo are administered, it is known that there is a risk of blood clots that block blood vessels (arterial thromboembolic events), which can cause a heart attack or stroke. Since a small amount of the medicine enters the bloodstream, there is a theoretical risk of these episodes after injection of Vabysmo into the eye.
Reporting of side effects
If you experience any side effects, talk to your doctor, even if you think they might be unrelated to the medicine. You can also report side effects directly through the national reporting system listed in Appendix V*. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Vabysmo
Your doctor, pharmacist, or nurse is responsible for storing this medicine and disposing of any unused product correctly. The following information is intended for healthcare professionals.
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label and carton after EXP. The expiry date is the last day of the month stated.
Store in a refrigerator (2°C - 8°C).
Do not freeze.
Keep the tray sealed in the original packaging to protect the pre-filled syringe from light.
The pre-filled syringe may be stored at room temperature, 20°C to 25°C, in the original packaging for up to 24 hours.
6. Container Contents and Additional Information
Vabysmo Composition
- The active ingredient is faricimab. One milliliter of injectable solution contains 120 mg of faricimab. Each prefilled syringe contains 21 mg of faricimab in 0.175 ml of solution. This provides a usable dose to release a single dose of 0.05 ml of solution containing 6 mg of faricimab.
- The other components are: L-histidine, 30% acetic acid (E 260), L-methionine, sodium chloride, sucrose, polysorbate 20 (E 432), water for injectable preparations (see Section 2 "Vabysmo contains sodium and polysorbate").
Product Appearance and Container Contents
Vabysmo 120 mg/ml injectable solution (injection) in a prefilled syringe is a clear to opalescent, transparent to yellow-brown solution.
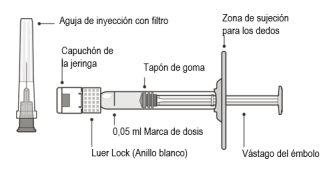
The container contains an injection needle with an ultra-fine wall filter (30 x ½ inch, 0.30 mm x 12.7 mm, 5 µm), along with a prefilled syringe for single use.
Marketing Authorization Holder and Manufacturer
Roche Registration GmbH
Emil-Barell-Strasse 1
79639
Grenzach-Wyhlen
Germany
Manufacturer
Roche Pharma AG
Emil-Barell-Strasse 1
79639
Grenzach-Wyhlen
Germany
You can request more information about this medication by contacting the local representative of the marketing authorization holder:
Belgium/Belgique/Belgien N.V. Roche S.A. Tel: +32 (0) 2 525 82 11 | Lithuania UAB "Roche Lietuva" Tel: +370 5 2546799 |
| Luxembourg/Luxemburg (See Belgium/Belgien) |
Czech Republic Roche s. r. o. Tel: +420 - 2 20382111 | Hungary Roche (Hungary) Kft. Tel: +36 - 1 279 4500 |
Denmark Roche Pharmaceuticals A/S Tlf: +45 - 36 39 99 99 | Malta (See Ireland) |
Germany Roche Pharma AG Tel: +49 (0) 7624 140 | Netherlands Roche Nederland B.V. Tel: +31 (0) 348 438050 |
Estonia Roche Eesti OÜ Tel: + 372 - 6 177 380 | Norway Roche Norge AS Tlf: +47 - 22 78 90 00 |
Greece Roche (Hellas) A.E. Τηλ: +30 210 61 66 100 | Austria Roche Austria GmbH Tel: +43 (0) 1 27739 |
Spain Roche Farma S.A. Tel: +34 - 91 324 81 00 | Poland Roche Polska Sp.z o.o. Tel: +48 - 22 345 18 88 |
France Roche Tél: +33 (0) 1 47 61 40 00 | Portugal Roche Farmacêutica Química, Lda Tel: +351 - 21 425 70 00 |
Croatia Roche d.o.o Tel: +385 1 4722 333 | Romania Roche România S.R.L. Tel: +40 21 206 47 01 |
Ireland Roche Products (Ireland) Ltd. Tel: +353 (0) 1 469 0700 | Slovenia Roche farmacevtska družba d.o.o. Tel: +386 - 1 360 26 00 |
Iceland Roche Pharmaceuticals A/S c/o Icepharma hf Sími: +354 540 8000 | Slovak Republic Roche Slovensko, s.r.o. Tel: +421 - 2 52638201 |
Italy Roche S.p.A. Tel: +39 - 039 2471 | Finland Roche Oy Puh/Tel: +358 (0) 10 554 500 |
| Sweden Roche AB Tel: +46 (0) 8 726 1200 |
Latvia Roche Latvija SIA Tel: +371 - 6 7039831 |
Date of Last Revision of this Prospectus:
Detailed information about this medication is available on the European Medicines Agency website: https://www.ema.europa.eu.
This information is intended only for healthcare professionals:
Instructions for use of the prefilled syringe:
Before Starting: | |
Read all instructions carefully before using Vabysmo. | |
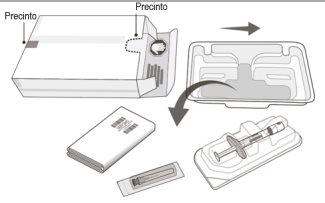
The Vabysmo container contains: | |
A sterile prefilled syringe in a sealed tray. The prefilled syringe is for single use. | |
An injection needle with a sterile filter, 30 x ½ inch, ultra-fine wall with an integrated filter in the connector. The injection needle with filter is for single use. | |
Use only the provided injection needle with filter for administration, as it has been designed to ensure safe ophthalmic use of the medication | |
Vabysmo should be stored in a refrigerator at temperatures between 2 °C and 8 °C. | |
Do notfreeze. | |
Allow Vabysmo to reach room temperature, between 20 °C and 25 °C, before proceeding with administration. | |
Before use, keep the sealed tray in the original packaging to protect the prefilled syringe from light. The prefilled syringe can be stored at room temperature in the original packaging for up to 24hours. | |
Vabysmo should be visually inspected before administration. | |
Do notuse the container if the seals have been tampered with. | |
Do notuse it if the container, prefilled syringe, or injection needle with filter is expired, damaged, or has been tampered with. | |
Do notuse it if the injection needle with filter is missing. | |
Do notremove the finger grip area from the syringe. | |
Do notuse it if the syringe cap has been removed from the Luer lock. | |
Do notuse it if particles are visible, if it appears cloudy, or if it is discolored. Vabysmo is a clear to opalescent, transparent to yellow-brown solution. |
Container Contents | ||
Figure A | ||
Product Description | ||
Figure B | ||
Remove the syringe from the syringe tray (step 1). All subsequent steps should be performed using aseptic techniques. | ||
Open the tray and remove the syringe cap | ||
1 | Remove the tray cover and aseptically remove the prefilled syringe. | |
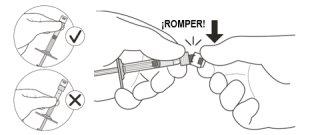
2 | Hold the syringe by the white ring and break the syringe cap (see Figure C). | |
Do nottwist the cap. | ||
Figure C | ||
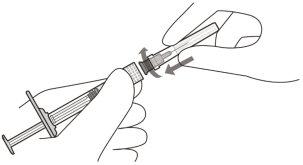
Attach the injection needle with filter | ||
3 | Aseptically remove the injection needle with filter from its container. | |
4 | Firmly and aseptically attach the injection needle with filter to the syringe Luer lock (see Figure D). | |
Figure D | Use only the provided injection needle with filter for administration | |
5 | Carefully remove the needle cap by pulling it straight off. | |
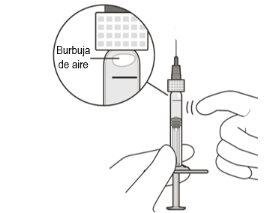
Remove Air Bubbles | ||
6 | Hold the syringe with the needle pointing upwards. Check the syringe to ensure there are no air bubbles. | |
7 | If there are air bubbles, gently tap the syringe with your finger until the bubbles rise to the top (see Figure E). | |
Figure E | ||
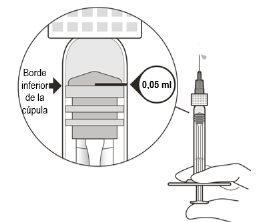
Adjust the Medication Dose and Remove Air | ||
8 | Hold the syringe at eye level and slowlypush the plunger rod until the lower edge of the rubber stopperis aligned with the 0.05 ml dose mark (see Figure F). This will remove the air and excess solution and adjust the dose to 0.05 ml. | |
Ensure that the injection is administered immediatelyafter dose preparation. | ||
Figure F | ||
Injection Procedure | ||
9 | The injection procedure should be performed under aseptic conditions. Inject slowlyuntil the rubber stopper reaches the bottom of the syringe to release the 0.05 ml volume. Do notrecap or separate the injection needle with filter from the syringe. Disposal of unused medication and all materials that have come into contact with it will be carried out in accordance with local regulations. |
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to VABYSMO 120 mg/mL solution for injection in pre-filled syringeDosage form: INJECTABLE, 120 mg/mLActive substance: faricimabManufacturer: Roche Registration GmbhPrescription requiredDosage form: INJECTABLE, 40 mg/mlActive substance: afliberceptManufacturer: Sandoz GmbhPrescription requiredDosage form: INJECTABLE, 40 mg/mlActive substance: afliberceptManufacturer: Sandoz GmbhPrescription required
Online doctors for VABYSMO 120 mg/mL solution for injection in pre-filled syringe
Discuss questions about VABYSMO 120 mg/mL solution for injection in pre-filled syringe, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions