
UPLIZNA 100 mg Concentrate for Solution for Infusion

How to use UPLIZNA 100 mg Concentrate for Solution for Infusion
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
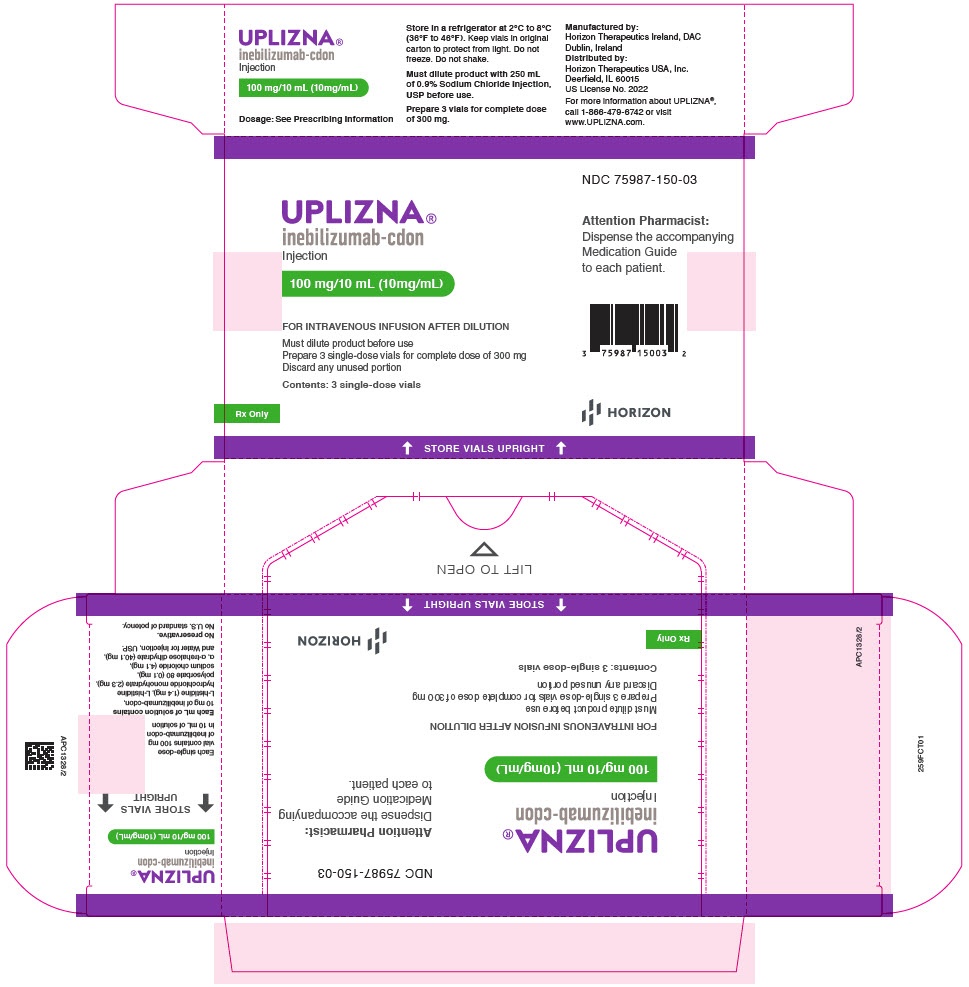
Uplizna 100 mg concentrate for solution for infusion
inebilizumab
This medicine is subject to additional monitoring, which will allow for quick identification of new safety information. You can help by reporting any side effects you may get. The last section of section 4 will tell you how to report side effects.
Read all of this leaflet carefully before you are given this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Uplizna and what is it used for
- What you need to know before you are given Uplizna
- How Uplizna is given
- Possible side effects
- Storage of Uplizna
- Contents of the pack and other information
1. What is Uplizna and what is it used for
Uplizna contains the active substance inebilizumab and belongs to a class of medicines called monoclonal antibodies. It is a protein that targets the antibody-producing cells in the immune system (the body's natural defenses) called B lymphocytes. Uplizna is used to reduce the risk of attacks in adults with a rare condition called neuromyelitis optica spectrum disorder (NMOSD), which affects the nerves in the eye and spinal cord. It is thought that the condition is caused by the immune system mistakenly attacking the body's nerves. Uplizna is given to patients with NMOSD whose B lymphocytes produce antibodies against aquaporin 4, a protein that plays an important role in nerve function.
2. What you need to know before you are given Uplizna
Do not use Uplizna
- if you are allergic to inebilizumab or any of the other ingredients of this medicine (listed in section 6).
- if you have a severe active infection such as hepatitis B.
- if you have active or untreated latent tuberculosis.
- if you have a history of progressive multifocal leukoencephalopathy (PML), a rare but serious brain infection caused by a virus.
- if you have been told you have serious immune system problems.
- if you have cancer.
Warnings and precautions
Tell your doctor, pharmacist, or nurse before you are given Uplizna if:
- you have or think you have an infection.
- you have ever taken, are taking, or are planned to take medicines that affect the immune system or other treatments for NMOSD. These medicines may increase your risk of getting an infection.
- you have ever had hepatitis B or are a hepatitis B virus carrier.
- you have recently received a vaccine or are planned to receive one. You should receive any required vaccines at least 2 weeks before starting treatment with Uplizna.
Infusion-related reactions
Uplizna may cause infusion-related reactions that can include headache, feeling sick (nausea), sleepiness, shortness of breath, fever, muscle pain, rash, or other symptoms. If symptoms occur, treatment may be interrupted or stopped.
Children and adolescents
This medicine must not be given to children and adolescents because it has not been studied in this population.
Other medicines and Uplizna
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines.
Pregnancy, breastfeeding, and fertility
If you are pregnant, think you may be pregnant, or plan to become pregnant, consult your doctor before you are given this medicine.
Pregnancy
Uplizna must not be used during pregnancy because the medicine may pass through the placenta and affect the baby. If you can become pregnant, you must use a contraceptive method (contraception) continuously once you start receiving Uplizna. If your doctor recommends stopping treatment, continue using your contraceptive method until 6 months after the last infusion.
Breastfeeding
It is not known whether Uplizna passes into breast milk. If you are breastfeeding, talk to your healthcare professional about the best way to feed your baby if you start treatment with Uplizna.
Driving and using machines
Uplizna is not expected to affect your ability to drive or use machines.
Uplizna contains sodium
This medicine contains 48 mg of sodium (a major component of table salt/cooking salt) in each infusion. This is equivalent to 2% of the maximum recommended daily intake of sodium for an adult.
3. How Uplizna is given
Uplizna is given by infusion (drip) into a vein under the supervision of a doctor with experience in treating patients with NMOSD.
The recommended dose is 300 mg.
The first dose is followed 2 weeks later by a second dose, and then one dose every 6 months.
You will be given other medicines between half an hour and one hour before the infusion to reduce the risk of you getting side effects. A doctor or nurse will monitor you during the infusion and for one hour after.
If you are unsure, consult your doctor again.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. Your doctor will discuss with you the possible side effects and explain the risks and benefits of Uplizna before treatment.
Serious side effects
The most seriousside effectsare infusion-related reactions and infections (see section 2). These side effects can occur at any time during treatment or even after your treatment has finished. You may experience more than one side effect at the same time. If you get an infusion-related reaction or an infection, call or consult your doctor immediately.
Other side effects
Very common(may affect more than 1 in 10 people)
- bladder infection
- infection in the nose, throat, sinuses, and/or lungs
- common cold
- flu
- joint pain
- back pain
- low immunoglobulin levels
Common(may affect up to 1 in 10 people)
- low white blood cell count, which sometimes occurs 4 weeks or more after the last dose of Uplizna
- sinusitis, usually caused by an infection
- pneumonia (lung infection)
- cellulitis, a potentially serious bacterial skin infection
- shingles (herpes zoster, a painful rash with blisters on one part of the body)
- reaction to Uplizna infusion (see Infusion-related reactions, above)
Uncommon(may affect up to 1 in 100 people)
- blood infection (sepsis), an extremely severe response to an infection
- progressive multifocal leukoencephalopathy (PML), a rare but serious brain infection caused by a virus
- abscess (an infection under the skin, usually caused by bacteria)
- bronchiolitis, a viral infection of the airways
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if it is possible side effects not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Uplizna
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton after EXP.
The expiry date is the last day of the month stated.
Store in a refrigerator (2°C - 8°C).
Store in the original package to protect from light. Do not freeze.
Do not use this medicine if you notice particles or a change in color.
6. Contents of the pack and other information
What Uplizna contains
- The active substance is inebilizumab.
- Each vial contains 100 mg of inebilizumab.
- The other ingredients are histidine, histidine hydrochloride monohydrate, polysorbate 80, sodium chloride, trehalose dihydrate, and water for injections.
Appearance and package contents
Uplizna 100 mg concentrate for solution for infusion is a clear to slightly opalescent, colorless to slightly yellow solution supplied in a carton containing 3 vials.
Marketing authorisation holder
Amgen Europe B.V.
Minervum 7061
4817 ZK Breda
Netherlands
Manufacturer
Horizon Therapeutics Ireland DAC
Pottery Road
Dun Laoghaire
Co. Dublin
A96 F2A8
Ireland
Manufacturer
Amgen NV
Telecomlaan 5-7
1831 Diegem
Belgium
You can request more information about this medicine from the local representative of the marketing authorisation holder:
België/Belgique/Belgien s.a. Amgen n.v. Tél/Tel: +32 (0)2 7752711 | Lietuva Amgen Switzerland AG Vilniaus filialas Tel. +370 5 219 7474 |
| Luxembourg/Luxemburg s.a. Amgen Belgique/Belgien Tél/Tel: +32 (0)2 7752711 |
Ceská republika Amgen s.r.o. Tel: +420 221 773 500 | Magyarország Amgen Kft. Tel.: +36 1 35 44 700 |
Danmark Amgen, filial af Amgen AB, Sverige Tlf.: +45 39617500 | Malta Amgen S.r.l. Italy Tel: +39 02 6241121 |
Deutschland Amgen GmbH Tel.: +49 89 1490960 | Nederland Amgen B.V. Tel: +31 (0)76 5732500 |
Eesti Amgen Switzerland AG Vilniaus filialas Tel: +372 586 09553 | Norge Amgen AB Tlf: +47 23308000 |
Ελλ?δα Amgen Ελλ?ς Φαρμακευτικ? Ε.Π.Ε. Τηλ: +30 210 3447000 | Österreich Amgen GmbH Tel: +43 (0)1 50 217 |
España Amgen S.A. Tel: +34 93 600 18 60 | Polska Amgen Biotechnologia Sp. z o.o. Tel.: +48 22 581 3000 |
France Amgen S.A.S. Tél: +33 (0)9 69 363 363 | Portugal Amgen Biofarmacêutica, Lda. Tel: +351 21 4220606 |
Hrvatska Amgen d.o.o. Tel: +385 (0)1 562 57 20 | România Amgen România SRL Tel: +4021 527 3000 |
Ireland Amgen Ireland Limited Tel: +353 1 8527400 | Slovenija AMGEN zdravila d.o.o. Tel: +386 (0)1 585 1767 |
Ísland Vistor hf. Sími: +354 535 7000 | Slovenská republika Amgen Slovakia s.r.o. Tel: +421 2 321 114 49 |
Italia Amgen S.r.l. Tel: +39 02 6241121 | Suomi/Finland Amgen AB, sivuliike Suomessa/Amgen AB, filial i Finland Puh/Tel: +358 (0)9 54900500 |
K?προς C.A. Papaellinas Ltd Τηλ: +357 22741 741 | Sverige Amgen AB Tel: +46 (0)8 6951100 |
Latvija Amgen Switzerland AG Rigas filiale Tel: +371 257 25888 |
Date of last revision of this leaflet:
Other sources of information
Detailed information on this medicine is available on the European Medicines Agency website: http://www.ema.europa.eu.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to UPLIZNA 100 mg Concentrate for Solution for InfusionDosage form: INJECTABLE PERFUSION, 120 mg (80 mg/kg) belimumabActive substance: belimumabManufacturer: Glaxosmithkline (Ireland) LimitedPrescription requiredDosage form: INJECTABLE, 200 mgActive substance: belimumabManufacturer: Glaxosmithkline (Ireland) LimitedPrescription requiredDosage form: INJECTABLE PERFUSION, 400 mg (80 mg/kg) belimumabActive substance: belimumabManufacturer: Glaxosmithkline (Ireland) LimitedPrescription required
Online doctors for UPLIZNA 100 mg Concentrate for Solution for Infusion
Discuss questions about UPLIZNA 100 mg Concentrate for Solution for Infusion, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions







