
BENLYSTA 200 mg Injectable Solution in a Pre-filled Pen

How to use BENLYSTA 200 mg Injectable Solution in a Pre-filled Pen
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Benlysta 200 mg solution for injection in pre-filled pen
belimumab
This medicine is subject to additional monitoring, which will allow for quick identification of new safety information. You can help by reporting any side effects you may get. The last section of section 4 will tell you how to report side effects.
Read all of this leaflet carefully before you start using this medicine, because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack and other information
- What is Benlysta and what is it used for
- What you need to know before you use Benlysta
- How to use Benlysta
- Possible side effects
- Storage of Benlysta
- Contents of the pack and other information
Step-by-step instructions for using the pre-filled pen
1. What is Benlysta and what is it used for
Benlysta as a subcutaneous injection is a medicine used to treat lupus(systemic lupus erythematosus, SLE) in adults (from 18 years of age) and children (from 5 to 18 years of age and with a minimum weight of 15 kg) whose disease remains very active despite standard treatment. Benlysta is also used in combination with other medicines to treat adults (from 18 years of age) with active lupus nephritis (kidney inflammation associated with lupus).
Lupus is a disease in which the immune system (the system that fights infections) attacks its own cells and tissues, causing inflammation and organ damage. It can affect any organ in the body and is thought to involve a type of white blood cells called B cells.
Benlysta contains belimumab(a monoclonal antibody). It reduces the number of B cells in your blood by blocking the action of BLyS, a protein that helps to prolong the survival of B cells and is found at high levels in people with lupus.
You will be given Benlysta along with your usual treatment for lupus.
2. What you need to know before you use Benlysta
Do not use Benlysta
- if you are allergicto belimumab or any of the other ingredients of this medicine (listed in section6).
- Tell your doctorif you think this may apply to you.
Warnings and precautions
Talk to your doctor before you start using Benlysta:
- if you have a current or chronic infectionor if you get infections often. Your doctor will decide if you can be given Benlysta
- if you are planning to be vaccinatedor have been vaccinated in the last 30 days. Some vaccines should not be given just before or during treatment with Benlysta
- if your lupus affects your nervous system
- if you have HIVor low levels of immunoglobulins
- if you have, or have had, hepatitis Bor C
- if you have had an organ transplantor a bone marrow transplantor stem cell transplant
- if you have had cancer
- if you have ever developed a severe skin rashor skin peeling, and/or mouth ulcersafter using Benlysta.
- Tell your doctorif you think any of these apply to you.
Depression and suicide
There have been reports of depression, suicidal thoughts and suicide attempts, including suicide during treatment with Benlysta. Tell your doctor if you have a history of these conditions. If you experience new symptoms or worsening of symptoms at any time:
- Contact your doctor or go immediately to the hospital.
If you feel depressed or have thoughts of self-harm or suicidal thoughts, it may be helpful to tell a family member or close friend and ask them to read this leaflet. You might ask them to tell you if they notice any changes in your mood or behaviour.
Severe skin reactions
There have been reports of Stevens-Johnson syndrome and toxic epidermal necrolysis associated with Benlysta treatment.
- Stop using Benlysta and seek medical attention immediatelyif you notice any of the symptoms described in section 4.
Be aware of important symptoms
People taking medicines that affect their immune system may be more prone to developing infections, including a rare but serious brain infection called progressive multifocal leukoencephalopathy (PML).
- Read the information “Increased risk of brain infection” in section 4 of this leaflet.
To improve the traceability of this medicine, you and your healthcare professional should record the batch number of Benlysta. It is recommended that you note this information in case it is needed in the future.
Children and adolescents
The Benlysta pre-filled pen for subcutaneous injection is not intended for use in the treatment of SLE in children under 5 years of age or weighing less than 15 kg.
The Benlysta pre-filled pen for subcutaneous injection is not intended for use in the treatment of lupus nephritis in children or adolescents under 18 years of age.
Other medicines and Benlysta
Tell your doctorif you are taking or have recently taken or might take any other medicines.
In particular, tell your doctor if you are being treated with medicines that affect your immune system, including any medicine that affects your B cells (to treat cancer or inflammatory diseases).
Using these medicines in combination with Benlysta may make your immune system less effective. This could increase the risk of you getting a serious infection.
Pregnancy and breastfeeding
Contraception for women who may become pregnant
- Usea reliable method of contraceptionwhile you are being treated with Benlysta and for at least 4 months after the last dose.
Pregnancy
Benlysta is not normally recommended during pregnancy.
- Tell your doctorif you are pregnant, think you may be pregnant or plan to become pregnant. Your doctor will decide if you can use Benlysta.
- If you become pregnantwhile you are being treated with Benlysta, tell your doctor.
Breastfeeding
Tell your doctorif you are breastfeeding. It is likely that Benlysta will pass into breast milk. Your doctor will advise whether you should stop treatment with Benlysta while you are breastfeeding or stop breastfeeding.
Driving and using machines
Benlysta may have effects that can affect your ability to drive or use machines.
Benlysta contains polysorbate80
This medicine contains 0.1 mg of polysorbate 80 in each pre-filled pen. Polysorbates may cause allergic reactions. Tell your doctor if you or your child have any known allergies.
Benlysta contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per dose; this is, essentially “sodium-free”.
3. How to use Benlysta
Follow the instructions for administration of this medicine exactly as told by your doctor or pharmacist. If you are not sure, ask your doctor or pharmacist again.
Benlysta should be injected under the skin following the schedule prescribed by your doctor.
How much to use
Systemic lupus erythematosus
Adults
The recommended dose is 200 mg (the entire contents of one pen) once a week.
Children and adolescents from 5 years of age
The recommended dose for children and adolescents from 5 years of age is based on body weight as follows:
Body weight | Recommended dose |
50 kg or more | 200 mg (the entire contents of one pen) once a week |
from 30 kg to less than 50 kg | 200 mg (the entire contents of one pen) once every 10 days |
from 15 kg to less than 30 kg | 200 mg (the entire contents of one pen) once every 2 weeks |
Lupus nephritis
Only adults
The recommended dose may vary.Your doctor will tell you the right dose for you, which may be:
- a dose of 200 mg (the entire contents of one pen) once a week
or
- a dose of 400 mg (the entire contents of two pens on one day) once a week for 4 weeks. After that, the recommended dose is 200 mg (the entire contents of one pen) once a week.
If you want to change your dosing day
Give a dose on the new day (even if the time since your last dose is less than usual). Continue with the new schedule from that day.
Injecting Benlysta
Your doctor or nurse will show you or your caregiver how to inject Benlysta. The first injection with the Benlysta pre-filled pen will be supervised by your doctor or nurse in the medical centre. After you have been trained on how to use the pen, your doctor or nurse will tell you when you or your caregiver can start giving the medicine without their supervision. Your doctor or nurse will also tell you what signs and symptoms to look out for when using Benlysta, as serious allergic reactions can occur (see "Allergic reactions"in section 4).
For children under 10 years of age, the Benlysta pre-filled pen must be injected by a doctor, nurse or qualified caregiver.
Benlysta is injected under the skin in the stomach area (abdomen) or in the top of the leg (thigh).
Benlysta subcutaneous injection must not be injected into a vein (by intravenous route).
Instructions for using the pre-filled pen are provided at the end of this leaflet.
If you use more Benlysta than you should
If this happens, contact your doctor or nurse immediately, who will monitor you for any signs or symptoms of side effects and treat these symptoms if necessary. If possible, show them the pack or this leaflet.
If you forget to use Benlysta
Inject the missed dose as soon as you remember and then continue with your normal weekly schedule as usual or start a new weekly schedule from the day you injected the missed dose.
If you do not realize you have missed a dose until it is time for your next dose, just inject your next dose as planned.
Stopping treatment with Benlysta
Your doctor will decide if you need to stop using Benlysta.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Stop using Benlysta and seek medical attention immediatelyif you notice any of the following symptoms of a severe skin reaction:
- red patches on the trunk, often with blisters in the centre, skin peeling, mouth ulcers, throat, nose, genitals and eyes. These severe skin reactions can be preceded by fever and flu-like symptoms (Stevens-Johnson syndrome and toxic epidermal necrolysis). These side effects have been reported with an unknown frequency (cannot be estimated from the available data).
Allergic reactions - get medical help immediately
Benlysta can cause an injection-related reaction or an allergic reaction (hypersensitivity). These are common side effects (may affect up to 1 in 10 people). They can occasionally be serious (uncommon, affecting up to 1 in 100 people) and life-threatening. It is more likely that these serious side effects will occur during the first or second day of treatment with Benlysta, but they can be delayed and occur several days later.
If you experience any of the following symptoms of an allergic reaction or injection-related reaction contact your doctor or nurse immediatelyorgo to the Emergency Department of your nearest hospital:
- swelling of the face, lips, mouth or tongue
- wheezing, difficulty breathing or troubled breathing
- skin rash
- itchy rash or hives.
In rare cases, less serious late reactions to Benlysta can also occur, usually 5 to 10 days after injection. These include symptoms such as skin rash, feeling unwell, tiredness, muscle pain, headache or swelling of the face.
If you experience these symptoms,especially if you notice two or more at the same time:
- Tell your doctor or nurse.
Infections
Benlysta may make you more likely to get infections, including urinary tract infections and respiratory infections. These infections are very common and may affect more than 1 in 10 people. Some infections can be serious and, in rare cases, can cause death.
If you have any of the following symptoms of an infection:
- fever and/or chills
- cough, breathing problems
- diarrhoea, vomiting
- burning sensation when urinating; frequent urination
- hot, red or painful skin or skin lesions on your body.
- Tell your doctor or nurse immediately.
Depression and suicide
There have been reports of depression, suicidal thoughts and suicide attempts during treatment with Benlysta. Depression may affect up to 1 in 10 people, suicidal thoughts or attempts may affect up to 1 in 100 people. If you feel depressed, have thoughts of self-harm or other concerning thoughts or are depressed and notice that you are getting worse or developing new symptoms:
- Contact your doctor or go immediately to the hospital.
Increased risk of brain infection
Medicines that weaken your immune system, such as Benlysta, may increase the risk of getting a rare but serious and potentially life-threatening brain infection called progressive multifocal leukoencephalopathy(PML).
Symptomsof PML include:
- memory loss
- problems thinking
- difficulty talking or walking
- loss of vision.
- Tell your doctor immediatelyif you have any of these symptoms or similar problems that have lasted for several days.
If you already had these symptoms before starting treatment with Benlysta:
- Tell your doctor immediatelyif you experience any change in these symptoms.
Other possible side effects:
Very common side effects
May affect more than 1 in 10people:
- bacterial infections (see “Infections” above).
Common side effects
May affect up to 1 in 10people:
- high temperature or fever
- reactions at the injection site, for example: skin rash, redness, itching or swelling of the skin where Benlysta has been injected
- itchy rash, hives, skin rash
- low levels of white blood cells (can be seen in blood tests)
- infection of the nose, throat or stomach
- pain in hands or feet
- migraine
- feeling unwell, diarrhoea.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Benlysta
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and on the label after EXP. The expiry date refers to the last day of that month.
Store in a refrigerator (2°C - 8°C).
Do not freeze.
Store in the original package to protect from light.
A single pre-filled pen can be stored at room temperature (up to 25°C) for a maximum of 12 hours, provided it is protected from light. Once removed from the refrigerator, the pen must be used within 12 hours or discarded.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Contents and Additional Information
Composition of Benlysta
The active ingredient is belimumab.
Each ml of the pre-filled pen contains 200 mg of belimumab.
The other components are arginine hydrochloride, histidine, histidine monohydrochloride, polysorbate 80 (E 433), sodium chloride, and water for injection. See section 2 for more information on polysorbate 80 and sodium content.
Appearance and Container Contents of the Product
Benlysta is supplied as a solution, from colorless to slightly yellow, in a single-use 1 ml pre-filled pen.
Available in packs of 1 or 4 pre-filled pens per pack and multiple packs containing 12 pre-filled pens (3 packs of 4 pre-filled pens).
Only some pack sizes may be marketed.
Marketing Authorization Holder
GlaxoSmithKline (Ireland) Limited
12 Riverwalk
Citywest Business Campus
Dublin 24
Ireland
Manufacturer
GlaxoSmithKline Manufacturing S.P.A
Strada Provinciale Asolana, 90
43056 San Polo di Torrile
Parma
Italy
You can request more information about this medicine by contacting the local representative of the marketing authorization holder:
België/Belgique/Belgien GlaxoSmithKline Pharmaceuticals s.a./n.v. Tel: + 32 (0) 10 85 52 00 | Lietuva GlaxoSmithKline Trading Services Limited Tel: + 370 80000334 |
| Luxembourg/Luxemburg GlaxoSmithKline Pharmaceuticals s.a./n.v. Belgique/Belgien Tel: + 32 (0) 10 85 52 00 |
Ceská republika GlaxoSmithKline s.r.o. Tel: + 420 222 001 111 | Magyarország GlaxoSmithKline Trading Services Limited Tel.: + 36 80088309 |
Danmark GlaxoSmithKline Pharma A/S Tlf: + 45 36 35 91 00 | Malta GlaxoSmithKline Trading Services Limited Tel: + 356 80065004 |
Deutschland GlaxoSmithKline GmbH & Co. KG Tel.: + 49 (0)89 36044 8701 | Nederland GlaxoSmithKline BV Tel: + 31 (0)33 2081100 |
Eesti GlaxoSmithKline Trading Services Limited Tel: + 372 8002640 | Norge GlaxoSmithKline AS Tlf: + 47 22 70 20 00 |
Ελλáδα GlaxoSmithKline Μονοπρóσωπη A.E.B.E. Τηλ: + 30 210 68 82 100 | Österreich GlaxoSmithKline Pharma GmbH Tel: + 43 (0)1 97075 0 |
España GlaxoSmithKline, S.A. Tel: + 34 900 202 700 | Polska GSK Services Sp. z o.o. Tel.: + 48 (0)22 576 9000 |
France Laboratoire GlaxoSmithKline Tél.: + 33 (0)1 39 17 84 44 | Portugal GlaxoSmithKline – Produtos Farmacêuticos, Lda. Tel: + 351 21 412 95 00 |
Hrvatska GlaxoSmithKline Trading Services Limited Tel:+ 385 800787089 | România GlaxoSmithKline Trading Services Limited Tel: + 40 800672524 |
Ireland GlaxoSmithKline (Ireland) Limited Tel: + 353 (0)1 4955000 | Slovenija GlaxoSmithKline Trading Services Limited Tel: + 386 80688869 |
Ísland Vistor ehf. Sími: +354 535 7000 | Slovenská republika GlaxoSmithKline Trading Services Limited Tel: + 421 800500589 |
Italia GlaxoSmithKline S.p.A. Tel: + 39 (0)45 7741111 | Suomi/Finland GlaxoSmithKline Oy Puh/Tel: + 358 (0)10 30 30 30 |
Κúπρος GlaxoSmithKline Trading Services Limited Τηλ: + 357 80070017 | Sverige GlaxoSmithKline AB Tel: + 46 (0)8 638 93 00 |
Latvija GlaxoSmithKline Trading Services Limited Tel: + 371 80205045 | |
Date of Last Revision of this Leaflet:
Other Sources of Information
Detailed information on this medicine is available on the European Medicines Agency website: https://www.ema.europa.eu, and on the website of the Spanish Agency for Medicines and Health Products (AEMPS) (http://www.aemps.gob.es/).
--------------------------------------------------------------------------------------------------------------------
Step-by-Step Instructions for Using the Pre-filled Pen
Once a Week:for adults and children from 5 to less than 18 years of age and weighing 50 kg or more.
Once Every 10 Days:for children from 5 to less than 18 years of age and weighing 30 kg to less than 50 kg.
Once Every Two Weeks:for children from 5 to less than 18 years of age and weighing 15 kg to less than 30 kg.
Read These Sections First
Follow these instructions on how to use the pre-filled pen correctly. Not following these instructions may affect the proper functioning of the pre-filled pen. Additionally, you will need to receive training on how to use the pre-filled pen.
Benlysta is onlyfor subcutaneousadministration (under the skin).
To improve the traceability of this medicine, you and your healthcare professional should record the batch number of Benlysta. It is recommended that you note this information in case it is required in the future.
Storage
- Keep refrigerated until 30 minutes before use.
- Store in the original packaging to protect it from light
- Keep out of sight and reach of children.
- Keep away from heat and direct sunlight.
- Do notfreeze. If the pre-filled pen has been frozen, do notuse the pen even if it has been thawed.
- Do notuse and do notstore it again in the refrigerator if it has been left at room temperature for more than 12 hours.
Precautions
- The pre-filled pen should only be used once and then discarded.
- Do notshare your Benlysta pre-filled pen with another person.
- Do notshake.
- Do notuse if it has been dropped onto a hard surface.
- Do notremove the ring cover until just before injection.
Parts of the Benlysta Pre-filled Pen
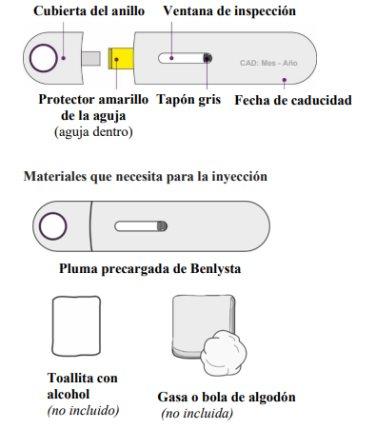

 Gather and Review the Materials
Gather and Review the Materials
Gather the Materials
- Remove a sealed tray containing the pre-filled pen from the refrigerator.
- Place the remaining pre-filled pens back in the refrigerator
- Find a comfortable, well-lit, and clean surface and place the following materials within reach:
- Benlysta pre-filled pen
- alcohol swab (not included in the packaging)
- cotton ball or pad (not included in the packaging)
- container with a tight-fitting lid for disposal of the pen (not included in the packaging).
- Do notperform the injection if you do not have all the mentioned materials.
Remove the Pre-filled Pen
- Peel off the film from the corner of the tray (Figure 1).
Figure1
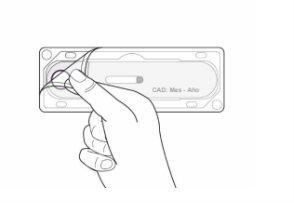
- Holding the center of the pre-filled pen (near the inspection window), carefully remove the pre-filled pen from the tray (Figure 2).
Figure2
Check the Expiration Date
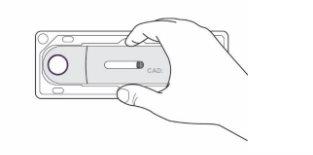
- Check the expiration date on the pre-filled pen (Figure 3).
Figure3
- Do not usethe pen if the expiration date has already passed.
- Prepare and Inspect the Pre-filled Pen
Allow it to Reach Room Temperature
- Let the pen stand at room temperature for 30 minutes (Figure 4). Injecting Benlysta when it is cold may take longer and may be uncomfortable.
Figure4

- Do notheat the pen in any other way. For example, in the microwave, hot water, or direct sunlight.
- Do notremove the ring cover during this step.
Inspect the Benlysta Solution
- Look at the inspection window to check that the Benlysta solution is from colorless to slightly yellow (Figure 5).
- It is normal to see one or more air bubbles in the solution.
Figure5
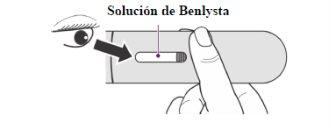
- Do notuse the pen if the solution appears cloudy, discolored, or has particles.
- Choose and Clean the Injection Site
Choose the Injection Site
- Choose a place for the injection (abdomen or thigh) as shown in Figure 6.
Figure6
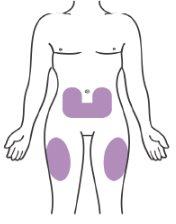
- If you need 2 injections to complete your dose, leave at least 5 cm (2 inches) between each injection if you use the same area.
- Do notalways inject in the same spot. This is to avoid the skin becoming hardened.
- Do notinject in areas where the skin is sensitive, bruised, red, or hard.
- Do notinject around 5 cm from the navel.
Clean the Injection Site
- Wash your hands.
- Clean the area where you will put the injection with an alcohol swab (Figure 7). Let the skin air dry.
Figure7
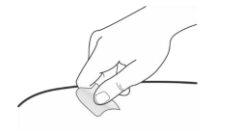
- Do nottouch this area again before administering the injection.
- Prepare for the Injection
Remove the Cover.
- Do notremove the cover until immediately before the injection.
- Remove the cover by pulling it or twisting it. The cover can be twisted clockwise or counterclockwise (Figure 8).
Figure8
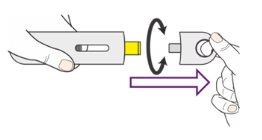
- Do notput the cover back on the pen.
Place the Pen
- Hold the pen comfortably so that you can see the inspection window. This is important so that you can confirm that a complete dose has been administered (Figure 9).
Figure9
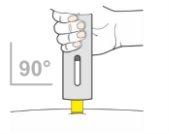
- If necessary, smooth out the injection site by pulling or stretching the skin.
- Place the pen directly on the injection site (at a 90° angle). Make sure the yellow needle protector is against the skin.
- Inject Benlysta and Check
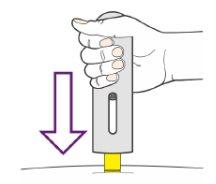
Start the Injection
- Press the pen firmly down on the injection site as far as it will go and hold it (Figure 10).
- This will insert the needle and start the injection.
Figure10
- You may hear a first "click" at the start of the injection. You will see the purple indicator start to move through the inspection window (Figure 11).
Figure11
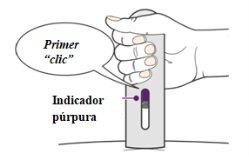
Complete the Injection
- Continue holding the pen down until the purple indicator has stopped moving.
- You may hear a second "click" a few seconds before the purple indicator stops moving (Figure 12).
Figure12
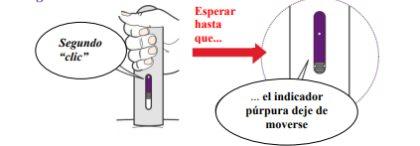
- The injection may take up to 15 seconds to complete.
- When the injection is complete, lift the pen off the injection site.
Check the Injection Site
There may be a small amount of blood at the injection site.
- If necessary, press a cotton ball or pad over the injection site.
- Do notrub the injection site.
- Dispose of the Used Pen
- Do notput the cover back on the pen.
- Dispose of the used pen and cover in a container with a tight-fitting lid.
- Ask your doctor or pharmacist for information on how to properly dispose of a used pen or a container of used pens.
- Do notrecycle or throw the pen or the container of used pens in household waste.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to BENLYSTA 200 mg Injectable Solution in a Pre-filled PenDosage form: INJECTABLE PERFUSION, 120 mg (80 mg/kg) belimumabActive substance: belimumabManufacturer: Glaxosmithkline (Ireland) LimitedPrescription requiredDosage form: INJECTABLE PERFUSION, 400 mg (80 mg/kg) belimumabActive substance: belimumabManufacturer: Glaxosmithkline (Ireland) LimitedPrescription requiredDosage form: INJECTABLE PERFUSION, 150 mgActive substance: ublituximabManufacturer: Neuraxpharm Pharmaceuticals S.L.Prescription required
Online doctors for BENLYSTA 200 mg Injectable Solution in a Pre-filled Pen
Discuss questions about BENLYSTA 200 mg Injectable Solution in a Pre-filled Pen, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions







