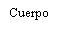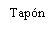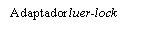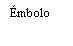
TWINRIX ADULTOS, PRE-FILLED SYRINGE SUSPENSION FOR INJECTION

Ask a doctor about a prescription for TWINRIX ADULTOS, PRE-FILLED SYRINGE SUSPENSION FOR INJECTION

How to use TWINRIX ADULTOS, PRE-FILLED SYRINGE SUSPENSION FOR INJECTION
Introduction
Package Leaflet: Information for the user
Twinrix Adult, Suspension for injection in a pre-filled syringe
Vaccine (HAB) (adsorbed) against hepatitis A (inactivated) and hepatitis B (ADNr)
Read all of this leaflet carefully before you start receiving this vaccine,because it contains important information for you.
- Keep this leaflet, as you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This vaccine has been prescribed for you only, and you should not pass it on to others.
- If you experience any side effects, talk to your doctor or pharmacist, even if they are not listed in this leaflet. See section 4.
Contents of the pack
- What is Twinrix Adult and what is it used for
- What you need to know before you receive Twinrix Adult
- How to administer Twinrix Adult
- Possible side effects
- Storage of Twinrix Adult
- Contents of the pack and further information
1. What is Twinrix Adult and what is it used for
Twinrix Adult is a vaccine used in adults and adolescents from 16 years of age to prevent two diseases: hepatitis A and hepatitis B. The vaccine works by making the body produce its own protection (antibodies) against these diseases.
- Hepatitis A: Hepatitis A is an infectious disease that can affect the liver. This disease is caused by the hepatitis A virus. Hepatitis A can be transmitted from person to person through food and drink, or by swimming in water contaminated with sewage. The symptoms of hepatitis A start 3 to 6 weeks after coming into contact with the virus. These include nausea (discomfort), fever, and pain. After several days, the whites of the eyes and skin may turn yellow (jaundice). The severity and type of symptoms can vary. Young children may not develop jaundice. Most people recover completely, but the disease is usually severe enough for patients to be ill for about a month.
- Hepatitis B: Hepatitis B is caused by the hepatitis B virus. It causes inflammation of the liver. The virus is found in body fluids such as blood, semen, vaginal secretions, or saliva (sputum) of infected people.
Vaccination is the best way to protect yourself against these diseases. None of the components of the vaccine are infectious.
2. What you need to know before you receive Twinrix Adult
Twinrix Adult must not be administered if:
- you are allergic to:
- the active substances or any of the other components of this vaccine (listed in section 6)
- neomycin.
Signs of an allergic reaction may include skin rash with itching, difficulty breathing, and swelling of the face or tongue.
- you have previously had an allergic reaction to any vaccine against hepatitis A and hepatitis B
- you have a severe infection with fever (over 38°C). A minor infection, such as a cold, should not be a problem for vaccination, but tell your doctor first.
Warnings and precautions
Talk to your doctor or pharmacist before receiving Twinrix Adult if:
- you have had any health problems after receiving a vaccine in the past
- you have a weakened immune system due to a disease or medication
- you have any bleeding problems or bruise easily.
Before or after any injection, fainting (especially in adolescents) may occur, so you should tell your doctor or nurse if you have fainted after receiving an injection in the past.
In obese people, a low response to the vaccine has been observed, possibly without achieving protection against hepatitis A. A low response to the vaccine, possibly without achieving protection against hepatitis B, has also been observed in older subjects, in men more than in women, in smokers, in obese people, and in people with chronic diseases, or those receiving medication. Your doctor may recommend that you have a blood test after completing the vaccination cycle to check if you have achieved a satisfactory response. If not, your doctor will indicate the possibility of needing additional doses.
Other medicines and Twinrix Adult
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before receiving this vaccine.
It is not known if Twinrix Adult passes into breast milk; however, it is not expected to cause problems for breastfed babies.
Twinrix Adult contains neomycin and sodium
Tell your doctor if you have had an allergic reaction to neomycin (an antibiotic).
This vaccine contains less than 1 mmol of sodium (23 mg) per dose; i.e., it is essentially "sodium-free".
3. How to administer Twinrix Adult
You will receive a total of three injections over a period of 6 months. Each injection will be given on a separate visit. The first dose will be given on the chosen date. The other two doses will be given one month and six months after the first dose.
- First dose: on the chosen date
- Second dose: 1 month later
- Third dose: 6 months after the first dose
A total of 3 doses of Twinrix Adult can also be given over 1 month. This vaccination schedule can only be given to adults who need rapid protection (e.g., travelers). The first dose will be given on the chosen date. The other two doses will be given 7 and 21 days after the first dose. A fourth dose is recommended 12 months later.
- First dose: on the chosen date
- Second dose: 7 days later
- Third dose: 21 days after the first dose
- Fourth dose: 12 months after the first dose
Your doctor will inform you if additional doses and future booster doses are needed.
As indicated in section 2, a low response to the vaccine, possibly without achieving protection against hepatitis B, is more frequent in older subjects, in men more than in women, in smokers, in obese people, and in people with chronic diseases, or those receiving medication. Your doctor may recommend that you have a blood test after completing the vaccination cycle to check if you have achieved a satisfactory response. If not, your doctor will indicate the possibility of needing additional doses.
If you miss one of the scheduled injections, talk to your doctor to schedule another visit.
Make sure you complete the full vaccination cycle of three injections. Otherwise, you may not be fully protected against the diseases.
Your doctor will administer the Twinrix Adult injection into the upper arm muscle.
The vaccine should not be injected subcutaneously (deeply) or intramuscularly into the buttock, as protection may be lower.
The vaccine should never be injected into a vein.
If you have any further questions on the use of this vaccine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this vaccine can cause side effects, although not everybody gets them.
The following side effects may occur:
Very common(may occur in more than 1 in 10 doses of vaccine): headache, pain and redness at the injection site, fatigue.
Common(may occur in up to 1 in 10 doses of vaccine): diarrhea, nausea, swelling, bruising, or itching at the injection site, general malaise.
Uncommon(may occur in up to 1 in 100 doses of vaccine): dizziness, vomiting, stomach pain, muscle pain, upper respiratory tract infection, fever of 37.5°C or higher.
Rare(may occur in up to 1 in 1,000 doses of vaccine): swelling of the lymph glands in the neck, armpit, or groin (lymphadenopathy), loss of skin sensation to pain or touch (hypoesthesia), tingling sensation (paresthesia), skin rash, itching, joint pain, loss of appetite, low blood pressure, flu-like symptoms such as fever, sore throat, runny nose, cough, and chills.
Very rare(may occur in up to 1 in 10,000 doses of vaccine):
Among the side effects that occurred very rarely during clinical trials, routine use of the vaccine, or with individual hepatitis A and hepatitis B vaccines, the following are included: reduced platelet count, which increases the risk of bleeding or bruising (thrombocytopenia), purple or brown-red spots visible through the skin (thrombocytopenic purpura), brain inflammation or infection (encephalitis), degenerative brain disease (encephalopathy), nerve inflammation (neuritis), numbness or weakness of the arms and legs (neuropathy), paralysis, seizures, or fits, swelling of the face, mouth, or throat (angioneurotic edema), purple or purple-red swelling of the skin (lichen planus), severe skin rashes (erythema multiforme), hives, joint inflammation, muscle weakness, infection around the brain that can cause severe headache with stiff neck and sensitivity to light (meningitis), inflammation of some blood vessels (vasculitis), abnormal liver laboratory test results, multiple sclerosis, spinal cord inflammation (myelitis), drooping eyelids and sinking of the muscles on one side of the face (facial paralysis), temporary nerve inflammation, which causes pain, weakness, and paralysis of the limbs and often progresses to the chest and face (Guillain-Barré syndrome), eye nerve disease (optic neuritis), immediate pain at the injection site, itching, and burning sensation.
Severe allergic reactions (anaphylaxis, anaphylactoid reactions, and serum sickness-like reaction) can also occur very rarely (in up to 1 in 10,000 doses of the vaccine). Some signs of severe allergic reactions may include skin rashes with itching or blisters, swelling of the eyes and face, difficulty breathing or swallowing, sudden drop in blood pressure, and loss of consciousness. These reactions can occur before leaving the doctor's office. In any case, if you experience any of these symptoms, you should see a doctor immediately.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist, even if they are not listed in this leaflet. You can also report them directly through the Spanish Medicines Agency's online reporting system, https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Twinrix Adult
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the pack. The expiry date is the last day of the month stated.
Store in a refrigerator (between 2°C and 8°C).
Store in the original packaging to protect from light.
Do not freeze. Freezing destroys the vaccine.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Content and Additional Information
Composition of Twinrix Adult
The active ingredients are:
Hepatitis A virus (inactivated)1,2 720 ELISA Units
Hepatitis B surface antigen3,4 20 micrograms
1Produced in human diploid cells (MRC-5)
2Adsorbed on hydrated aluminum hydroxide 0.05 milligrams Al3+
3Produced by recombinant DNA technology in yeast cells (Saccharomyces cerevisiae)
4Adsorbed on aluminum phosphate 0.4 milligrams Al3+
The other components of Twinrix Adult are: sodium chloride and water for injections.
Appearance ofTwinrix Adultand Container Content
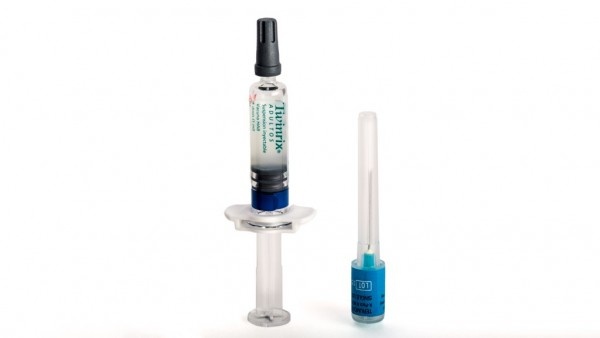
Injectable suspension in a pre-filled syringe.
Twinrix Adult is a white, slightly milky liquid.
Twinrix Adult is available in a pre-filled syringe with or without separate needles, in pack sizes of 1, 10, and 25.
Only some pack sizes may be marketed.
Marketing Authorization Holder and Manufacturer
GlaxoSmithKline Biologicals s.a.
Rue de l’Institut 89
B-1330 Rixensart
Belgium
For further information on this medicinal product, please contact the local representative of the marketing authorization holder:
België/Belgique/Belgien GlaxoSmithKline Pharmaceuticals SA/NV Tel: + 32 10 85 52 00 | Lietuva GlaxoSmithKline Biologicals SA Tel: +370 80000334 |
България GlaxoSmithKline Biologicals SA Tel: + 359 80018205 | Luxembourg/Luxemburg GlaxoSmithKline Pharmaceuticals SA/NV Tel: + 32 10 85 52 00 |
Česká republika GlaxoSmithKline s.r.o. Tel: + 420 2 22 00 11 11 | Magyarország GlaxoSmithKline Biologicals SA Tel: + 36 80088309 |
Danmark GlaxoSmithKline Pharma A/S Tlf: + 45 36 35 91 00 | Malta GlaxoSmithKline Biologicals SA Tel: + 356 80065004 |
Deutschland GlaxoSmithKline GmbH & Co. KG Tel: + 49 (0)89 360448701 | Nederland GlaxoSmithKline BV Tel: + 31 (0)33 2081100 |
Eesti GlaxoSmithKline Biologicals SA Tel: +372 8002640 | Norge GlaxoSmithKline AS Tlf: + 47 22 70 20 00 |
Ελλάδα GlaxoSmithKline Μονοπρόσωπη A.E.B.E. Τηλ: + 30 210 68 82 100 | Österreich GlaxoSmithKline Pharma GmbH Tel: + 43 (0)1 970750 |
España GlaxoSmithKline, S.A. Tel: + 34 900 202 700 | Polska GSK Services Sp. z o.o. Tel: + 48 (22) 576 9000 |
France Laboratoire GlaxoSmithKline Tél: + 33 (0) 1 39 17 84 44 Hrvatska GlaxoSmithKline Biologicals SA Tel: + 385 800787089 | Portugal Smith Kline & French Portuguesa - Produtos Farmacêuticos, Lda Tel: + 351 21 412 95 00 România GlaxoSmithKline Biologicals SA Tel: +40 800672524 |
Ireland GlaxoSmithKline (Ireland) Ltd Tel: + 353 (0)1 495 5000 | Slovenija GlaxoSmithKline Biologicals SA Tel: + 386 80688869 |
Ísland Vistor hf Sími: +354 535 7000 | Slovenská republika GlaxoSmithKline Biologicals SA Tel: + 421 800500589 |
Italia GlaxoSmithKline S.p.A. Tel: + 39 (0)45 774 1111 | Suomi/Finland GlaxoSmithKline Oy Puh/Tel: + 358 10 30 30 30 |
Κύπρος GlaxoSmithKline Biologicals SA Τηλ: + 357 80070017 | Sverige GlaxoSmithKline AB Tel: + 46 (0)8 638 93 00 |
Latvija GlaxoSmithKline Biologicals SA Tel: + 371 80205045 | United Kingdom(Northern Ireland) GlaxoSmithKline Biologicals SA Tel: +44 (0)800 221 441 |
Date of Last Revision of this Leaflet:04/2023
Other Sources of Information
Detailed information on this medicinal product is available on the European Medicines Agency website http://www.ema.europa.eu and on the website of the Spanish Agency for Medicines and Health Products (AEMPS) (http://www.aemps.gob.es/).
---------------------------------------------------------------------------------------------------------------------
This information is intended only for healthcare professionals:
During storage, a fine white deposit with a clear, colorless layer on top may be observed.
The vaccine should be resuspended before use. Once resuspended, the vaccine will have a uniform, white, and turbid appearance.
Resuspension of the Vaccine to Obtain a Uniform, White, and Turbid Suspension
The vaccine should be resuspended by following the steps below.
- Hold the syringe upright with your hand closed.
- Shake the syringe by turning it upside down and then back upright.
- Repeat this action vigorously for at least 15 seconds.
- Inspect the vaccine again:
- If the vaccine appears as a uniform, white, and turbid suspension, it is ready to use (it should not have a clear appearance).
- If the vaccine still does not appear as a uniform, white, and turbid suspension, turn it upside down and then back upright for at least another 15 seconds and then inspect again.
Before administration, the vaccine should be visually inspected for any foreign particles and/or abnormal physical appearance. If any of these circumstances are observed, do not administer the vaccine.
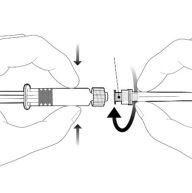
Instructions for the Pre-filled Syringe after Resuspension
| Hold the syringe by the body, not by the plunger. Unscrew the syringe cap by turning it counterclockwise. | |
| To insert the needle, connect the base to the Luer-Lock adapter and turn it a quarter turn clockwise until it clicks. Do not pull the plunger out of the syringe body. If this happens, do not administer the vaccine. |
Disposal of Waste
Disposal of unused medicinal products and all materials that have come into contact with them should be done in accordance with local regulations.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to TWINRIX ADULTOS, PRE-FILLED SYRINGE SUSPENSION FOR INJECTIONDosage form: INJECTABLE, UnknownActive substance: combinationsManufacturer: Glaxosmithkline BiologicalsPrescription requiredDosage form: INJECTABLE, UnknownActive substance: combinationsManufacturer: Glaxosmithkline BiologicalsPrescription requiredDosage form: INJECTABLE, UnknownActive substance: combinationsManufacturer: Glaxosmithkline BiologicalsPrescription required
Alternatives to TWINRIX ADULTOS, PRE-FILLED SYRINGE SUSPENSION FOR INJECTION in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to TWINRIX ADULTOS, PRE-FILLED SYRINGE SUSPENSION FOR INJECTION in Ukraine
Online doctors for TWINRIX ADULTOS, PRE-FILLED SYRINGE SUSPENSION FOR INJECTION
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for TWINRIX ADULTOS, PRE-FILLED SYRINGE SUSPENSION FOR INJECTION – subject to medical assessment and local rules.