TRYDONIS 87/5/9 micrograms pressurized inhalation solution

How to use TRYDONIS 87/5/9 micrograms pressurized inhalation solution
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Trydonis 87micrograms/5micrograms/9micrograms inhalation solution in a pressurized container
dipropionate of beclometasone/formoterol fumarate dihydrate/glycopyrronium
Read this package leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this package leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this package leaflet. See section 4.
Contents of the package leaflet
- What is Trydonis and what is it used for
- What you need to know before you use Trydonis
- How to use Trydonis
- Possible side effects
- Storage of Trydonis
- Contents of the pack and other information
1. What is Trydonis and what is it used for
Trydonis is a medicine to help you breathe that contains three active substances:
- dipropionate of beclometasone,
- formoterol fumarate dihydrate and
- glycopyrronium.
The dipropionate of beclometasone belongs to a group of medicines called corticosteroids, which act by reducing inflammation and irritation in the lungs.
Formoterol and glycopyrronium are medicines called long-acting bronchodilators. They work in different ways to relax the muscles of the airways, helping to widen them and making it easier for you to breathe.
Regular treatment with these three active substances helps to relieve and prevent symptoms such as difficulty breathing, wheezing, and coughing in adult patients with chronic obstructive pulmonary disease (COPD). Trydonis may reduce the exacerbations (worsening of symptoms) of COPD. COPD is a serious, long-term disease in which the airways are blocked and the air sacs inside the lungs are damaged, making it difficult to breathe.
2. What you need to know before you use Trydonis
Do not use Trydonis:
If you are allergic to dipropionate of beclometasone, formoterol fumarate dihydrate, and/or glycopyrronium or to any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions:
Trydonis is used as a maintenance treatment for chronic obstructive pulmonary disease. Do not use this medicine to treat a sudden attack of difficulty breathing or wheezing.
If your breathing worsens:
If you experience worsening of difficulty breathing or wheezing (breathing with a whistling sound) immediately after inhaling the medicine, stop the treatment with the Trydonis inhaler and use your quick-acting "rescue" inhaler immediately. You should contact your doctor right away. Your doctor will assess your symptoms and, if necessary, may change your treatment.
See also section 4. Possible side effects.
If your lung disease worsens:
If your symptoms worsen or are difficult to control (e.g., if you are using another "rescue" inhaler more frequently) or if your "rescue" inhaler does not improve your symptoms, contact your doctor immediately. It may be that your lung disease is worsening and your doctor may need to prescribe a different treatment.
Consult your doctor or pharmacist before you start using Trydonis:
If you are in any of the above situations, inform your doctor before you start using Trydonis.
If you have or have had any health problems or allergies, or if you are unsure whether you can use Trydonis, consult your doctor or pharmacist before you start using the inhaler.
If you are already using Trydonis
If you are using Trydonis or high doses of other inhaled corticosteroids for long periods of time and you experience a stressful situation (e.g., if you are taken to the hospital after an accident, have a severe injury, or before an operation), you may need more medicine. In such situations, your doctor may need to increase your dose of corticosteroids to cope with the stress and may prescribe them in the form of tablets or injections.
Contact your doctor if you experience blurred vision or other visual disturbances.
Children and adolescents
Do not give this medicine to children and adolescents under 18 years of age.
Other medicines and Trydonis
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines. This includes medicines similar to Trydonis used for your lung disease.
Some medicines may increase the effects of Trydonis, so your doctor will monitor you closely if you are taking these medicines (including some for HIV: ritonavir, cobicistat).
Do not use this medicine with a beta-blocker medicine(used to treat certain heart problems such as angina or to lower blood pressure) unless your doctor has chosen a beta-blocker that does not affect your breathing. Beta-blockers (including beta-blockers in eye drops) may reduce the effects of formoterol or make them disappear. On the other hand, the use of other beta2 agonist medicines (which work in the same way as formoterol) may increase the effects of formoterol.
The use of Trydonis with:
- medicines to treat
- abnormal heart rhythms (quinidine, disopyramide, procainamide),
- allergic reactions (antihistamines),
- symptoms of depression or mental disorders such as monoamine oxidase inhibitors (e.g., phenelzine and isocarboxazid), tricyclic antidepressants (e.g., amitriptyline and imipramine), and phenothiazines
may cause some changes in the electrocardiogram (ECG, heart tracing). They may also increase the risk of heart rhythm disorders (ventricular arrhythmias).
- medicines to treat Parkinson's disease (levodopa), medicines to treat an underactive thyroid gland (levothyroxine), medicines that contain oxytocin (which causes uterine contractions), and alcohol may increase the likelihood of formoterol's adverse effects on the heart.
- monoamine oxidase inhibitors (MAOIs), including medicines with similar properties such as furazolidone and procarbazine, used to treat mental disorders, may cause an increase in blood pressure.
- medicines to treat heart disease (digoxin) may cause a decrease in potassium levels in the blood. This may increase the likelihood of abnormal heart rhythms.
- other medicines used to treat COPD (theophylline, aminophylline, or corticosteroids) and diuretics may also cause a drop in potassium levels.
- certain anesthetics may increase the risk of abnormal heart rhythms.
- disulfiram, a medicine used in the treatment of people with alcoholism (alcohol problems), or metronidazole, an antibiotic to treat infections in your body, may cause adverse effects (e.g., nausea, vomiting, stomach pain) due to the small amount of alcohol contained in Trydonis.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
You should only use Trydonis during pregnancy if your doctor advises you to. It is recommended to avoid the use of Trydonis during childbirth due to the inhibitory effects of formoterol on uterine contractions.
Do not use Trydonis during breastfeeding. You and your doctor must decide whether to stop breastfeeding or stop the treatment, considering the benefit of breastfeeding to your child and the benefit of the treatment to you.
Driving and using machines
It is unlikely that Trydonis will affect your ability to drive or use machines.
Trydonis contains ethanol
Trydonis contains 8.856 mg of alcohol (ethanol) per actuation, which is equivalent to 17.712 mg per dose of two actuations. The amount in two actuations of this medicine is equivalent to less than 1 ml of wine or beer. The small amount of alcohol in this medicine does not produce any noticeable effect.
3. How to use Trydonis
Follow the administration instructions for this medication exactly as indicated by your doctor or pharmacist. If in doubt, consult your doctor or pharmacist again.
The recommended dose is two doses in the morning and two doses at night.
If you think the medication is not being very effective, consult your doctor.
If you have been using another inhaler that contained beclometasone dipropionate previously, consult your doctor, as the effective dose of beclometasone dipropionate in Trydonis for the treatment of COPD may be lower than that of other inhalers.
Route of administration
Trydonis is for inhalation use only.
You should inhale the medication through the mouth, which carries the medication directly to the lungs.
This medication is in a pressurized container within a plastic inhaler with a mouthpiece.
Trydonis is available in three package sizes:
- a package that provides 60 doses
- a package that provides 120 doses
- a package that provides 180 doses
If you have been prescribed a package that provides 60doses or 120doses
There is a counter on the back of the inhaler, which indicates how many doses are left. Each time you press the pressurized container, a dose of medication will be released, and the counter will subtract one unit. Avoid dropping the inhaler, as this could cause the counter to decrement.
If you have been prescribed a package that provides 180doses
There is an indicator on the back of the inhaler, which indicates how many doses are left. Each time you press the pressurized container, a dose of medication will be released, and the counter will turn slightly. The number of remaining doses is displayed in intervals of 20. Avoid dropping the inhaler, as this could cause the counter to decrement.
Checking the inhaler
Before using the inhaler for the first time, you must check it in the following way to ensure it is working properly.
- Depending on the package size you have been prescribed, check that the dose counter reading is 61 or 121, and the dose indicator reading is 180
- Remove the protective cap from the mouthpiece
- Hold the inhaler upright with the mouthpiece at the bottom
- Point the mouthpiece away from you and firmly press the pressurized container to release a dose
- Check the dose counter or dose indicator. If you are checking the inhaler for the first time, the counter should indicate:
60
| 120
| 180
|
|
|
|
How to use the inhaler
Stand or sit upright to perform the inhalation.
IMPORTANT: do not perform steps 2 to 5 too quickly.
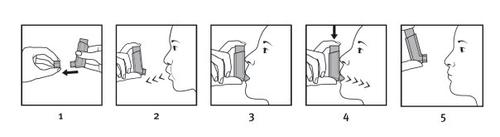
- Remove the protective cap from the mouthpiece and check that the mouthpiece is clean, i.e., free of dust and dirt.
- Exhale slowly and deeply as much as possible to empty your lungs.
- Hold the inhaler straight with the mouthpiece facing down and place the mouthpiece between your teeth without biting it. Then, place your lips around the mouthpiece, with your tongue flat underneath it.
- Inhale slowly and deeply through your mouth to fill your lungs with air (this should take about 4-5 seconds). Just after starting to inhale, firmly press the top of the pressurized container to release a dose.
- Hold your breath for as long as you can and finally remove the inhaler from your mouth and exhale slowly. Do not blow air through the inhaler.
- Check that the dose counter (60/120 doses) has subtracted one unit or that the dose indicator (180 doses) has turned slightly.
To take the second dose, hold the inhaler upright for about half a minute and then repeat steps 2 to 5.
If some of the gas escapes from the top of the inhaler or from the corner of your lips, it means that Trydonis will not reach your lungs as it should. Take another dose following the instructions, starting again from step 2.
After use, replace the protective cap.
To avoid a fungal infection in the mouth and throat, rinse your mouth or gargle with water without swallowing or brush your teeth after each use of the inhaler.
When to obtain a new inhaler
You should obtain a replacement when the counter or indicator shows the number 20. Stop using the inhaler when the counter or inhaler shows a 0, as any remaining medication in the inhaler may be insufficient to provide a complete dose.
If you have weakness to grasp, you may find it easier to hold the inhaler with both hands: place your two index fingers on the top of the inhaler and your two thumbs on the bottom of the inhaler.
If you find it difficult to use the inhaler while starting to inhale, you can use the AeroChamber Plus spacer device. Consult your doctor or pharmacist about this device.
It is essential that you read the leaflet provided with the AeroChamber Plus spacer device and follow its instructions for use and cleaning carefully.
Cleaning the Trydonis inhaler
You should clean the inhaler once a week.
- Do not remove the pressurized container from the inhaler, and do not use water or other liquids to clean it.
- Remove the protective cap from the mouthpiece by pulling it to separate it from the inhaler.
- Clean the inside and outside of the mouthpiece and inhaler with a clean, dry cloth or paper towel.
- Replace the mouthpiece cap.
If you use more Trydonis than you should
It is essential that you take the dose as indicated by your doctor. Do not exceed the prescribed dose without consulting your doctor first.
If you use more Trydonis than you should, the adverse effects described in section 4 may occur.
Tell your doctor if you have used more Trydonis than you should and if you experience any of these symptoms. Your doctor may want to perform some blood tests.
If you forget to use Trydonis
Use it as soon as you remember. If it is almost time for the next dose, do not take the missed dose, but only the next dose at the correct time. Do not double the dose.
If you interrupt treatment with Trydonis
It is essential to use Trydonis every day. Do not interrupt treatment with Trydonis or reduce the dose, even if you feel better or do not have symptoms. If you want to do this, consult your doctor.
If you have any further questions about the use of this medication, ask your doctor or pharmacist.
4. Possible side effects
Like all medications, this medication can cause side effects, although not everyone will experience them.
There is a risk of worsening breathing difficulties and wheezing immediately after using Trydonis, known as paradoxical bronchospasm (may affect up to 1 in 1,000 people). If this occurs, you should stop treatment with Trydonis and use your quick-acting "rescue" inhaler immediately to treat breathing difficulties and wheezing. You should contact your doctor immediately.
Tell your doctor immediately
- if you experience allergic reactions such as skin allergies, hives, itching of the skin, rash (may affect up to 1 in 100 people), skin redness, swelling of the skin or mucous membranes, especially of the eyes, face, lips, and throat (may affect up to 1 in 1,000 people).
- if you experience pain or discomfort in the eyes, blurred vision, halos, or colored images associated with red eyes. These may be signs of an acute narrow-angle glaucoma attack (may affect up to 1 in 10,000 people).
Tell your doctor if you notice any of the following symptoms during the use of Trydonis, as they may be due to a lung infection (may affect up to 1 in 10 people):
- fever or chills
- increased mucus production, change in mucus color
- increased coughing or difficulty breathing
Possible side effects are listed below by frequency.
Frequent(may affect up to 1 in 10 people):
- sore throat
- nasal secretion or congestion and sneezing
- fungal infections of the mouth. Rinsing your mouth or gargling with water and brushing your teeth immediately after inhalation can help prevent these side effects
- hoarseness
- headache
- urinary tract infection
Uncommon(may affect up to 1 in 100 people):
|
|
Rare(may affect up to 1 in 1,000 people):
|
|
Very rare(may affect up to 1 in 10,000 people):
- low level of certain blood cells called platelets
- feeling of choking or difficulty breathing
- swelling of the hands and feet
- growth delay in children and adolescents
Frequency not known(cannot be estimated from the available data):
- blurred vision
The use of inhaled corticosteroids in high doses for a prolonged period may cause, in very rare cases, effects on the body:
- problems with the functioning of the adrenal glands (adrenal suppression)
- decrease in bone mineral density (thinning of the bones)
- clouding of the lens of the eye (cataract)
Trydonis does not contain a high-dose inhaled corticosteroid, but your doctor may want to check your cortisol levels in the blood from time to time.
The following side effects may also occur when using inhaled corticosteroids in high doses for a prolonged period, but their frequency is currently not known (cannot be estimated from the available data):
- depression
- feeling of worry, nervousness, overexcitement, or irritability
These effects are more likely in children.
Reporting side effects
If you experience any side effects, consult your doctor or pharmacist, even if they are possible side effects not listed in this leaflet. You can also report them directly through the Spanish Medicines Monitoring System for Human Use https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medication.
5. Storage of Trydonis
Keep this medication out of sight and reach of children.
Do not use this medication after the expiration date shown on the label and carton after CAD/EXP. The expiration date is the last day of the month indicated.
Do not freeze.
Do not expose to temperatures above 50°C.
Do not puncture the pressurized container.
Before dispensing:
Store in a refrigerator (between 2°C and 8°C).
After dispensing (after receiving this medication from your pharmacist):
Pressurized container of 60 puffs: | Store the inhaler below 25°C for a maximum of 2 months. |
Pressurized container of 120 (taken from a single or multiple package) and 180 puffs: | Store the inhaler below 25°C for a maximum of 4 months. |
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of the packaging and any unused medication. This will help protect the environment.
6. Container contents and additional information
Trydonis composition
The active ingredients are: beclometasone dipropionate, formoterol fumarate dihydrate, and glycopyrronium.
Each delivered dose (the dose that comes out of the mouthpiece) contains 87 micrograms of beclometasone dipropionate, 5 micrograms of formoterol fumarate dihydrate, and 9 micrograms of glycopyrronium (in the form of 11 micrograms of glycopyrronium bromide).
Each measured dose (the dose that comes out of the valve) contains 100 micrograms of beclometasone dipropionate, 6 micrograms of formoterol fumarate dihydrate, and 10 micrograms of glycopyrronium (in the form of 12.5 micrograms of glycopyrronium bromide).
The other ingredients are: anhydrous ethanol (see section 2), hydrochloric acid, propellant: norflurane.
This medicinal product contains fluorinated greenhouse gases.
Each 60-puff inhaler contains 6.481 g of norflurane (HFC-134a) which corresponds to 0.009 tons of CO2 equivalent (global warming potential GWP = 1430).
Each 120-puff inhaler contains 10.37 g of norflurane (HFC-134a) which corresponds to 0.015 tons of CO2 equivalent (global warming potential GWP = 1430).
Each 180-puff inhaler contains 14.259 g of norflurane (HFC-134a) which corresponds to 0.02 tons of CO2 equivalent (global warming potential GWP = 1430).
Appearance of the product and container contents
Trydonis is an inhalation solution in a pressurized container.
Trydonis is presented in a pressurized container (aluminum-coated), with a metering valve. The pressurized container is inserted in a plastic inhaler. This incorporates a mouthpiece with a plastic protective cap and a dose counter (containers with 60 and 120 doses) or a dose indicator (containers with 180 doses).
Each container contains a pressurized container that provides 60 doses, 120 doses, or 180 doses. Additionally, there are multiple containers that contain 240 doses (2 pressurized containers with 120 doses each) or 360 doses (3 pressurized containers with 120 doses each).
Only some container sizes may be marketed.
Marketing authorization holder
Chiesi Farmaceutici S.p.A.
Via Palermo 26/A
43122 Parma
Italy
Manufacturers
Chiesi Farmaceutici S.p.A.
Via San Leonardo 96
43122 Parma
Italy
Chiesi SAS
Rue Faraday
ZA des Gailletrous
41260 La Chaussée Saint Victor
France
Chiesi Pharmaceuticals GmbH
Gonzagagasse 16/16
1010 Wien
Austria
You can request more information about this medicinal product by contacting the local representative of the marketing authorization holder:
Belgium Chiesi sa/nv Tel: + 32 (0)2 788 42 00 | Lithuania Chiesi Pharmaceuticals GmbH Tel: + 43 1 4073919 |
| Luxembourg Chiesi sa/nv Tel: + 32 (0)2 788 42 00 |
Czech Republic Chiesi CZ s.r.o. Tel: + 420 261221745 | Hungary Chiesi Hungary Kft. Tel.: + 36-1-429 1060 |
Denmark Chiesi Pharma AB Tlf: + 46 8 753 35 20 | Malta Chiesi Farmaceutici S.p.A. Tel: + 39 0521 2791 |
Germany Chiesi GmbH Tel: + 49 40 89724-0 | Netherlands Chiesi Pharmaceuticals B.V. Tel: + 31 88 501 64 00 |
Estonia Chiesi Pharmaceuticals GmbH Tel: + 43 1 4073919 | Norway Chiesi Pharma AB Tlf: + 46 8 753 35 20 |
Greece Chiesi Hellas AEBE Τηλ: + 30 210 6179763 | Austria Chiesi Pharmaceuticals GmbH Tel: + 43 1 4073919 |
Spain Laboratorios BIAL, S.A. Tel: + 34 91 562 41 96 | Poland Chiesi Poland Sp. z.o.o. Tel.: + 48 22 620 1421 |
France Chiesi S.A.S. Tél: + 33 1 47688899 | Portugal Chiesi Farmaceutici S.p.A. Tel: + 39 0521 2791 |
Croatia Chiesi Pharmaceuticals GmbH Tel: + 43 1 4073919 | Romania Chiesi Romania S.R.L. Tel: + 40 212023642 |
Ireland Chiesi Farmaceutici S.p.A. Tel: + 39 0521 2791 | Slovenia CHIESI SLOVENIJA, d.o.o. Tel: + 386-1-43 00 901 |
Iceland Chiesi Pharma AB Sími: +46 8 753 35 20 | Slovakia Chiesi Slovakia s.r.o. Tel: + 421 259300060 |
Italy Chiesi Italia S.p.A. Tel: + 39 0521 2791 | Finland Chiesi Pharma AB Puh/Tel: +46 8 753 35 20 |
Cyprus Chiesi Farmaceutici S.p.A. Τηλ: + 39 0521 2791 | Sweden Chiesi Pharma AB Tel: +46 8 753 35 20 |
Latvia Chiesi Pharmaceuticals GmbH Tel: + 43 1 4073919 |
Date of last revision of this leaflet: November 2024
Other sources of information
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu.
- Country of registration
- Average pharmacy price74.35 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to TRYDONIS 87/5/9 micrograms pressurized inhalation solutionDosage form: PULMONARY INHALATION, 172 micrograms/5 micrograms/9 microgramsActive substance: formoterol, glycopyrronium bromide and beclometasoneManufacturer: Chiesi Farmaceutici S.P.A.Prescription requiredDosage form: PULMONARY INHALATION, 87/5/9 micrograms/actuationActive substance: formoterol, glycopyrronium bromide and beclometasoneManufacturer: Chiesi Farmaceutici S.P.A.Prescription requiredDosage form: PULMONARY INHALATION, 88 micrograms/ 5 micrograms/ 9 microgramsActive substance: formoterol, glycopyrronium bromide and beclometasoneManufacturer: Chiesi Farmaceutici S.P.A.Prescription required
Online doctors for TRYDONIS 87/5/9 micrograms pressurized inhalation solution
Discuss questions about TRYDONIS 87/5/9 micrograms pressurized inhalation solution, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions