
TRIMBOW 88/5/9 micrograms INHALATION POWDER

How to use TRIMBOW 88/5/9 micrograms INHALATION POWDER
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Trimbow 88micrograms/5micrograms/9micrograms powder for inhalation
beclometasone dipropionate/formoterol fumarate dihydrate/glycopyrronium
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What Trimbow is and what it is used for
- What you need to know before you use Trimbow
- How to use Trimbow
- Possible side effects
- Storing Trimbow
- Contents of the pack and other information
1. What Trimbow is and what it is used for
Trimbow is a medicine that helps you breathe and contains three active substances:
- beclometasone dipropionate,
- formoterol fumarate dihydrate and
- glycopyrronium.
Beclometasone dipropionate belongs to a group of medicines called corticosteroids, which act by reducing inflammation and irritation in the lungs.
Formoterol and glycopyrronium are medicines called long-acting bronchodilators. They work in different ways to relax the muscles of the airways, helping to widen them and making it easier for you to breathe.
Regular treatment with these three active substances helps to relieve and prevent symptoms such as difficulty breathing, wheezing and coughing in adult patients with chronic obstructive pulmonary disease (COPD). Trimbow can reduce the exacerbations (worsening of symptoms) of COPD. COPD is a serious, long-term disease where the airways are blocked and the air sacs in the lungs are damaged, making it difficult to breathe.
2. What you need to know before you use Trimbow
Do not use Trimbow
If you are allergic to beclometasone dipropionate, formoterol fumarate dihydrate and/or glycopyrronium or to any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Trimbow is used as a maintenance treatment for chronic obstructive pulmonary disease. Do not use this medicine to treat a sudden attack of difficulty breathing or wheezing.
If your breathing gets worse
If, just after inhaling the medicine, you experience worsening of difficulty breathing or wheezing (breathing with a whistling sound), stop the treatment with the Trimbow inhaler and use your quick-acting "rescue" inhaler immediately. You should contact your doctor immediately. Your doctor will assess your symptoms and, if necessary, may change your treatment.
See also section 4. Possible side effects.
If your COPD gets worse
If your symptoms get worse or are difficult to control (e.g. if you are using another "rescue" inhaler more often) or if your "rescue" inhaler does not improve your symptoms, go to your doctor immediately. It may be that your COPD is getting worse and your doctor may need to prescribe a different treatment for you.
Consult your doctor or pharmacist before starting to use Trimbow:
- if you have any heart problems, such as angina (chest pain), a recent heart attack (myocardial infarction), heart failure, narrowing of the heart arteries (coronary artery disease), heart valve disease or any other heart abnormality, or if you have a disease called hypertrophic obstructive cardiomyopathy (also known as HOCM, a disease where the heart muscle is abnormal).
- if you have heart rhythm disorders, such as an irregular heartbeat, a fast pulse or palpitations or if you have been told that your electrocardiogram (ECG) is abnormal.
- if you have narrowing of the arteries (also known as arteriosclerosis), if you have high blood pressure or if you have an aneurysm (an abnormal bulge in the wall of a blood vessel).
- if you have an overactive thyroid gland.
- if you have low levels of potassium in your blood (hypokalaemia). The combination of Trimbow with certain other medicines for the lungs or medicines such as diuretics (medicines that make the body lose water, to treat heart disease or high blood pressure) may cause a marked decrease in potassium levels in the blood. Therefore, your doctor may want to check your potassium levels in your blood from time to time.
- if you have any liver or kidney disease.
- if you have diabetes. High doses of formoterol may increase blood sugar levels, so you may need some additional blood tests to check your blood sugar levels when you start using this medicine and from time to time during treatment.
- if you have a tumour of the adrenal gland (known as phaeochromocytoma).
- if you are going to be given an anaesthetic. Depending on the type of anaesthetic, it may be necessary to stop treatment with Trimbow at least 12 hours before the anaesthetic.
- if you are receiving or have received treatment for tuberculosis (TB) or if you have a chest infection.
- if you have a certain eye problem called narrow-angle glaucoma.
- if you have difficulty urinating.
- if you have an infection in the mouth or throat.
If you are in any of these situations, tell your doctor before you start using Trimbow.
If you have or have had any other health problems or allergies or if you are unsure whether you can use Trimbow, consult your doctor or pharmacist before you start using the inhaler.
If you are already using Trimbow
If you are using Trimbow or high doses of other inhaled corticosteroids for long periods of time and you experience a stressful situation (e.g. if you are taken to hospital after an accident, have a serious injury or before an operation), you may need more medicine. In such situations, your doctor may need to increase your dose of corticosteroids to cope with the stress and may prescribe them in tablet or injection form.
Contact your doctor if you experience blurred vision or other visual disturbances.
Children and adolescents
Do not give this medicine to children and adolescents under 18 years of age.
Other medicines and Trimbow
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines. This includes medicines similar to Trimbow used for your lung disease.
Some medicines may increase the effects of Trimbow, so your doctor will monitor you closely if you are taking these medicines (including some for HIV: ritonavir, cobicistat).
Do not use this medicine with a beta-blocker medicine(used to treat certain heart problems such as angina or to lower blood pressure) unless your doctor has chosen a beta-blocker that does not affect your breathing. Beta-blockers (including beta-blockers in eye drops) may reduce the effects of formoterol or make them disappear. On the other hand, the use of other beta2 agonist medicines (which work in the same way as formoterol) may increase the effects of formoterol.
Using Trimbow with:
- medicines for treating
- abnormal heart rhythms (quinidine, disopyramide, procainamide),
- allergic reactions (antihistamines),
- symptoms of depression or mental disorders such as monoamine oxidase inhibitors (e.g. phenelzine and isocarboxazid), tricyclic antidepressants (e.g. amitriptyline and imipramine) and phenothiazines may cause some changes in the electrocardiogram (ECG). They may also increase the risk of heart rhythm disturbances (ventricular arrhythmias).
- medicines for treating Parkinson's disease (levodopa), medicines for treating an underactive thyroid gland (levothyroxine), medicines containing oxytocin (which causes uterine contractions) and alcohol may increase the likelihood of formoterol having adverse effects on the heart.
- monoamine oxidase inhibitors (MAOIs), including medicines with similar properties such as furazolidone and procarbazine, used to treat mental disorders, may cause an increase in blood pressure.
- medicines for treating heart disease (digoxin) may cause a decrease in potassium levels in the blood. This may increase the likelihood of abnormal heart rhythms.
- other medicines used to treat chronic obstructive pulmonary disease (theophylline, aminophylline or corticosteroids) and diuretics may also cause a drop in potassium levels.
- certain anaesthetics may increase the risk of abnormal heart rhythms.
Pregnancy and breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
You should only use Trimbow during pregnancy if your doctor advises you to. It is best to avoid using Trimbow during labour due to the inhibitory effects of formoterol on uterine contractions.
Do not use Trimbow during breast-feeding. You and your doctor should decide whether to stop breast-feeding or stop treatment with Trimbow, considering the benefit of breast-feeding for your child and the benefit of treatment for you.
Driving and using machines
Trimbow is unlikely to affect your ability to drive or use machines.
Trimbow contains lactose
Lactose contains small amounts of milk proteins, which may cause allergic reactions.
3. How to use Trimbow
Follow exactly the instructions for administration of this medicine given by your doctor or pharmacist. If you are in doubt, consult your doctor or pharmacist again.
The recommended dose is two inhalations in the morning and two inhalations in the evening.
If you think that the medicine is not being very effective, consult your doctor.
If you have been using another inhaler that contained beclometasone dipropionate before, consult your doctor, as the effective dose of beclometasone dipropionate in Trimbow for the treatment of chronic obstructive pulmonary disease may be lower than that of other inhalers.
Method of administration
Trimbow is for inhalation use only.
You should inhale the medicine through your mouth, which carries the medicine directly to the lungs.
Instructions for use
To view information about the contents of the pack, see section 6.
If the contents of the pack do not match those described in section 6, return the inhaler to the person who supplied it to you and get a new one.
- Donot remove the inhaler from the bag unless you intend to use it immediately.
- Always use the inhaler as directed.
- Keep the cover closed until you need to take a dose from the inhaler.
- When not in use, keep the inhaler in a clean and dry place.
- Donot attempt to dismantle the inhaler under any circumstances.
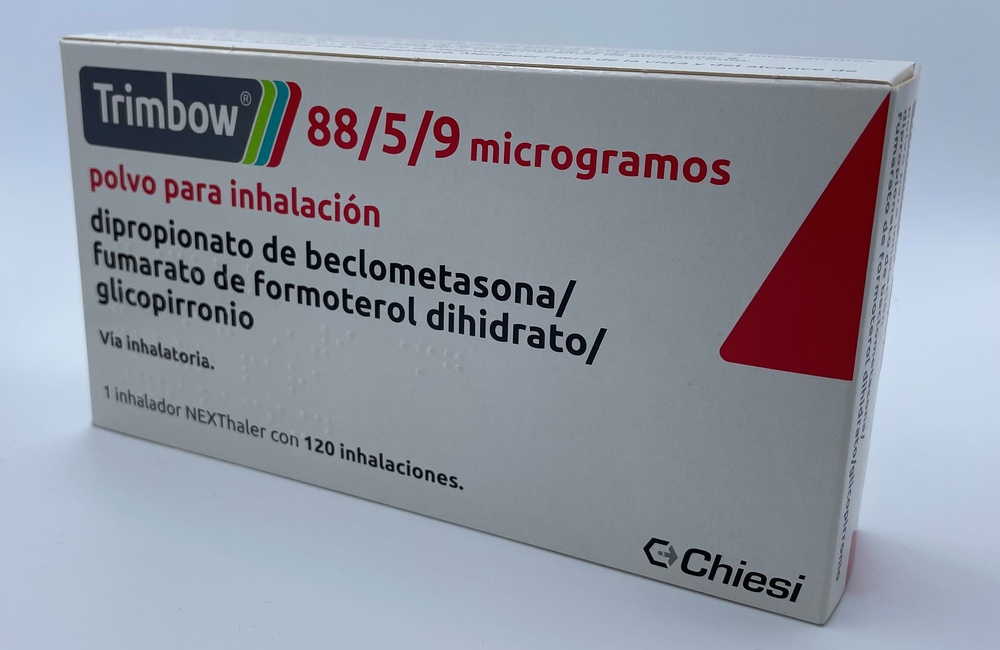
- Essential features of the inhaler
Taking a dose from the inhaler involves three steps: open, inhale, close.
- Before using a new inhaler
- Open the bag and remove the inhaler.
- Donot use the inhaler if the bag is not closed or is damaged; return it to the person who supplied it to you and get a new one.
- Use the label on the carton to note the date when you opened the bag.
- Inspect the inhaler.
- If the inhaler appears to be broken or damaged, return it to the person who supplied it to you and get a new one.
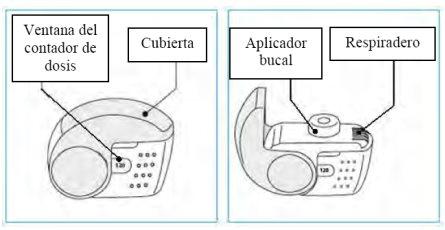
- Check the dose counter window. If the inhaler is new, you will see “120” in the dose counter window.
- Donot use the new inhaler if the number shown is less than “120”; return it to the person who supplied it to you and get a new one.
- How to use the inhaler
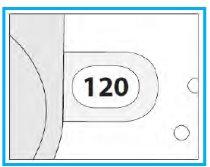
C.1. Open
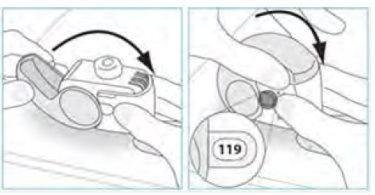
- Hold the inhaler firmly in an upright position.
- Check the number of doses left: any number between “1” and “120” indicates that there are doses left.
- If the dose counter window shows “0”, there are no doses left; dispose of the inhaler and get a new one.
- Open the cover fully.
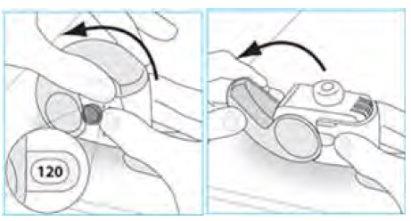
- Before inhaling, breathe out as slowly and as fully as you can.
- Donot breathe out through the inhaler.
C.2. Inhale
Whenever possible, stand or sit upright to perform the inhalation.
- Lift the inhaler, bring it to your mouth and put your lips around the mouthpiece.
- Donot cover the air vent when holding the inhaler.
- Donot inhale through the air vent.
- Take a strong and deep breath in through your mouth.
- You may notice a certain taste when taking the dose.
- You may hear or feel a click when taking the dose.
- Donot inhale through your nose.
- Donot remove the inhaler from your lips during inhalation.
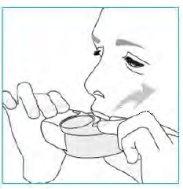
- Remove the inhaler from your mouth.
- Hold your breath for 5 to 10 seconds or for as long as you can.
- Breathe out slowly.
- Donot breathe out through the inhaler.
- If you are not sure whether you are receiving your dose correctly, contact your pharmacist or doctor.
C.3. Close
- Put the inhaler back in an upright position and close the cover fully.
- Check that the dose counter has gone down by one unit.
- If you are not sure whether the dose counter has gone down by one unit after an inhalation, wait until your next scheduled dose and take it as normal. Do not take an extra dose.
- If you need to take another dose, repeat steps C.1 to C.3.
- Cleaning
- The inhaler does not usually need to be cleaned.
- If necessary, you can clean the inhaler after use with a dry cloth or paper tissue.
- Donot clean the inhaler with water or other liquids. Keep it dry.
If you use more Trimbow than you should
It is important that you take the dose as instructed by your doctor. Do not exceed the prescribed dose without consulting your doctor first.
If you use more Trimbow than you should, the side effects described in section 4 may appear.
Tell your doctor if you have used more Trimbow than you should and if you experience any of these symptoms. Your doctor may want to perform some blood tests.
If you forget to use Trimbow
Use it as soon as you remember. If it is almost time for your next dose, do not take the missed dose, just take the next dose at the correct time. Do not take a double dose.
If you stop using Trimbow
It is important to use Trimbow every day. Do not stop treatment with Trimbow or reduce the dose, even if you feel better or do not have symptoms. If you want to do this, consult your doctor.
If you have any further questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible Adverse Effects
Like all medicines, this medicine can cause adverse effects, although not all people suffer from them.
There is a risk of worsening breathing difficulties and wheezing immediately after using Trimbow, known as paradoxical bronchospasm (may affect up to 1 in 1,000 people). If this occurs, you should discontinue treatment with Trimbow and use your rapid-acting "rescue" inhaler without delay to treat breathing difficulties and wheezing. You should contact your doctor immediately.
Inform your doctor immediately
- if you experience allergic reactions such as skin allergies, hives, itching of the skin, skin rash (may affect up to 1 in 100 people), skin redness, skin swelling or mucous membranes, especially of the eyes, face, lips, and throat (may affect up to 1 in 1,000 people).
- if you experience pain or discomfort in the eyes, blurred vision, visual halos, or colored images associated with red eyes. These may be signs of an acute narrow-angle glaucoma attack (may affect up to 1 in 10,000 people).
Inform your doctor if you notice any of the following symptoms during the use of Trimbow, as they may be due to a lung infection (may affect up to 1 in 10 people):
- fever or chills
- increased mucus production, change in mucus color
- increased coughing or difficulty breathing
The possible adverse effectsare listed below according to their frequency.
Frequent(may affect up to 1 in 10 people)
- throat pain
- nasal secretion or congestion and sneezing
- mouth fungal infections. Rinsing your mouth or gargling with water and brushing your teeth immediately after inhalation can help prevent these adverse effects
- hoarseness
- headache
- urinary tract infection
Uncommon(may affect up to 1 in 100 people)
|
|
Rare(may affect up to 1 in 1,000 people)
|
|
Very Rare(may affect up to 1 in 10,000 people)
- low level of certain blood cells called platelets
- feeling of suffocation or difficulty breathing
- swelling of the hands and feet
- growth delay in children and adolescents
Frequency Not Known(cannot be estimated from the available data)
- blurred vision
The use of inhaled corticosteroids in high doses for a prolonged period may cause, in very rare cases, effects on the body:
- problems with the functioning of the adrenal glands (adrenal suppression)
- decrease in bone mineral density (thinning of the bones)
- clouding of the eye lens (cataract)
Trimbow does not contain a high-dose inhaled corticosteroid, but your doctor may want to measure your cortisol levels in the blood from time to time.
The following adverse effects may also occur when inhaled corticosteroids are used in high doses for a prolonged period, but their frequency is currently not known (cannot be estimated from the available data):
- depression
- feeling of worry, nervousness, overexcitement, or irritability
These effects are more likely in children.
Reporting of Adverse Effects
If you experience any type of adverse effect, consult your doctor or pharmacist, even if it is a possible adverse effect that is not listed in this leaflet. You can also report them directly through the national reporting system included in Appendix V. By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Storage of Trimbow
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the label and carton after CAD/EXP. The expiry date is the last day of the month indicated.
Do not store above 25°C.
Store the inhaler in the original packaging to protect it from moisture and only remove it from the bag immediately before the first use.
After the first opening of the bag, the medicine should be used within 6 weeks and stored in a dry place. Use the adhesive label on the outer carton to note the date you opened the bag and attach this label to the bottom of the inhaler.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of the packaging and any unused medicine. This will help protect the environment.
6. Package Contents and Additional Information
Composition of Trimbow
The active ingredients are: beclometasone dipropionate, formoterol fumarate dihydrate, and glycopyrronium.
Each delivered dose (the dose that comes out of the mouthpiece) contains 88 micrograms of beclometasone dipropionate, 5 micrograms of formoterol fumarate dihydrate, and 9 micrograms of glycopyrronium (as 11 micrograms of glycopyrronium bromide).
Each measured dose contains 100 micrograms of beclometasone dipropionate, 6 micrograms of formoterol fumarate dihydrate, and 10 micrograms of glycopyrronium (as 12.5 micrograms of glycopyrronium bromide).
The other ingredients are: lactose monohydrate (see section 2) and magnesium stearate.
Appearance and Package Contents
Trimbow is a white to almost white inhalation powder.
It is supplied in a white plastic inhaler called NEXThaler with a gray mouthpiece cover and an inhalation counter.
Each inhaler is packaged in a closed protective bag.
Trimbow is available in packs containing one inhaler and multipacks containing two or three inhalers, each providing 120 inhalations (120, 240, or 360 inhalations).
Not all pack sizes may be marketed.
Marketing Authorization Holder
Chiesi Farmaceutici S.p.A.
Via Palermo 26/A
43122 Parma
Italy
Manufacturer
Chiesi Farmaceutici S.p.A.
Via San Leonardo 96
43122 Parma
Italy
Chiesi SAS
2 rue des Docteurs Alberto et Paolo Chiesi
41260 La Chaussée Saint Victor
France
You can request more information about this medicine by contacting the local representative of the marketing authorization holder:
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
| ||
|
Date of Last Revision of this Leaflet:December 2024
Other Sources of Information
Detailed information on this medicine is available on the European Medicines Agency website: http://www.ema.europa.eu.
- Country of registration
- Average pharmacy price74.35 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to TRIMBOW 88/5/9 micrograms INHALATION POWDERDosage form: PULMONARY INHALATION, 172 micrograms/5 micrograms/9 microgramsActive substance: formoterol, glycopyrronium bromide and beclometasoneManufacturer: Chiesi Farmaceutici S.P.A.Prescription requiredDosage form: PULMONARY INHALATION, 87/5/9 micrograms/actuationActive substance: formoterol, glycopyrronium bromide and beclometasoneManufacturer: Chiesi Farmaceutici S.P.A.Prescription requiredDosage form: PULMONARY INHALATION, 87 MICROGRAMS/5 MICROGRAMS/9 MICROGRAMSActive substance: formoterol, glycopyrronium bromide and beclometasoneManufacturer: Chiesi Farmaceutici S.P.A.Prescription required
Online doctors for TRIMBOW 88/5/9 micrograms INHALATION POWDER
Discuss questions about TRIMBOW 88/5/9 micrograms INHALATION POWDER, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions
















