TRAVATAN 40 micrograms/ml eye drops, solution

How to use TRAVATAN 40 micrograms/ml eye drops, solution
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
TRAVATAN 40 micrograms/ml eye drops, solution
travoprost
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist, even if you have read it in this leaflet. See section 4.
Contents of the pack
- What is TRAVATAN and what is it used for
- What you need to know before you use TRAVATAN
- How to use TRAVATAN
- Possible side effects
- Storing TRAVATAN
- Contents of the pack and other information
1. What is TRAVATAN and what is it used for
TRAVATAN contains travoprostwhich belongs to a group of medicines called prostaglandin analogues. It works by reducing the pressure in the eye. It can be used on its own or with other eye drops, for example beta blockers, which also reduce the pressure in the eye.
TRAVATAN is used to reduce high pressure in the eye in adults, adolescents, and children from 2 months of age. This pressure can lead to a condition called glaucoma.
2. What you need to know before you use TRAVATAN
Do not use TRAVATAN
- If you are allergic to travoprost or any of the other ingredients of this medicine (listed in section 6).
Tell your doctor if you are in this situation.
Warnings and precautions
- TRAVATAN may increasethe length, thickness, color and/or number of your eyelashes. Changes such as unusual hair growth on your eyelids or around your eyes have also been observed.
- TRAVATAN may change the color of your iris(the colored part of your eye). This change may be permanent. It can also cause a change in the color of the skin around your eyes.
- If you have had cataract surgery, consult your doctor before using TRAVATAN.
- If you suffer or have suffered from eye inflammation(iritis and uveitis), consult your doctor before using TRAVATAN.
- TRAVATAN may, in rare cases, cause shortness of breathor noisy breathingor increase the symptoms of asthma. If you are concerned about changes in your breathing while using TRAVATAN, consult your doctor as soon as possible.
- Travoprost may be absorbed through the skin. In caseof contactof the medicine with the skin, it should be removed by washingimmediately. This is especially important in pregnant or breast-feeding women.
- If you wear soft contact lenses, do not apply the drops while wearing them. After applying the drops, wait 15 minutes before putting your lenses back in.
Children and adolescents
TRAVATAN can be used in children from 2 months of age to less than 18 years of age with the same dose as in adults. The use of TRAVATAN is not recommended in children under 2 months of age.
Other medicines and TRAVATAN
Tell your doctor or pharmacist if you are using, have recently used or might use any other medicines.
Pregnancy, breast-feeding and fertility
Do not use TRAVATAN if you are pregnant. If you could be pregnant, tell your doctor. If you can become pregnant, use an effective method of birth control while using TRAVATAN.
Do not use TRAVATAN if you are breast-feeding. TRAVATAN may pass into breast milk.
Consult your doctor before using any medicine.
Driving and using machines
Immediately after applying TRAVATAN, you may notice that your vision becomes blurred. Do not drive or use machines until these effects have disappeared.
TRAVATAN contains hydrogenated castor oil and propylene glycol, which may cause skin reactions and irritation.
3. How to use TRAVATAN
Follow exactly the instructions of your doctor or the doctor treating the child. If you are unsure, consult your doctor, the doctor treating the child, or pharmacist.
The recommended dose is
One drop in the affected eye(s) once a day - in the evening.
TRAVATAN should only be applied in both eyes if your doctor has told you to do so. Continue the treatment for the whole period of time prescribed by your doctor or the doctor treating the child.
|
|
|
|
TRAVATAN should only be used as eye drops.
- Immediately before using a bottle for the first time, open the protective foil pouch, take out the bottle (figure 1), and write the date you opened it on the space provided on the carton.
- Wash your hands.
- Remove the cap.
- Hold the bottle upside down between your fingers.
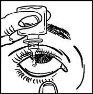
- Tilt your head or the child's head back gently. Gently pull the lower eyelid away from the eye to form a pocket, where the drop should fall (figure 2).
- Bring the tip of the bottle close to the eye. You may find it helpful to use a mirror.
- Do not touch the eye, eyelid, or surrounding areas with the dropper, because the drops could become contaminated.
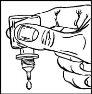
- Gently squeeze the bottle to release one drop of TRAVATAN at a time (figure 3).
- After using TRAVATAN, gently close your eyes, and press the edge of your eye next to your nose (figure 4)for at least 1 minute. This helps prevent TRAVATAN from getting into the rest of your body.
- If you are applying drops in both eyes, repeat the steps for the other eye.
- Close the bottle tightly after use.
- Use one bottle at a time. Do not open the pouch until you are ready to use the bottle.
If a drop falls outside the eye, try again.
If you or the child are using other eye medicines, such as eye drops or ointments, wait at least 5 minutes between applying TRAVATAN and the other eye medicines.
If you or the child get more TRAVATAN than needed
You can flush it out with warm water. Do not apply more drops until it is time for the next dose.
If you forget to use TRAVATAN
Continue with the next dose as planned. Do not apply a double doseto make up for a forgotten dose. Never apply more than one drop in the affected eye(s) in one day.
If you stop using TRAVATAN
Do not stop using TRAVATAN without consulting your doctor or the doctor treating the child, as the pressure in your eye or the child's eye will not be controlled, which could lead to loss of vision.
If you have any other questions about using this medicine, ask your doctor, the doctor treating the child, or pharmacist.
Continue
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
You can carry on using the treatment as usual, unless the side effects are serious. If they are, tell your doctor or pharmacist. Do not stop using TRAVATAN without talking to your doctor.
The following side effects have been seen with TRAVATAN:
Very common: may affect more than 1 in 10 people
Eye effects:redness of the eye.
Common: may affect up to 1 in 10 people
Eye effects:changes in iris color (the colored part of the eye), eye pain, discomfort in the eye, dry eye, itching in the eye, irritation in the eye.
Uncommon: may affect up to 1 in 100 people
Eye effects:corneal changes, eye inflammation, iris inflammation, inflammation inside the eye, inflammation with or without damage to the surface of the eye, sensitivity to light, eye discharge, eyelid inflammation, eyelid redness, swelling around the eye, itching of the eyelid, blurred vision, increased tear production, conjunctivitis (inflammation or infection of the conjunctiva), abnormal turning out of the lower eyelid, darkened vision, crusts on the eyelid, eyelash growth.
Other effects:increased allergic symptoms, headache, irregular heartbeat, cough, stuffy nose, sore throat, darkening of the skin around the eyes, darkening of the skin, abnormal hair texture, excessive hair growth.
Rare: may affect up to 1 in 1,000 people
Eye effects:flashes of light, eyelid eczema, abnormally positioned eyelashes that grow towards the eye, eye swelling, reduced vision, halos around lights, decreased sensation in the eye, inflammation of the eyelid glands, pigmentation inside the eye, increased pupil size, thickening of the eyelashes, change in eyelash color, tired eyes.
Other effects:viral eye infection, dizziness, bad taste, irregular or decreased heartbeat, increased or decreased blood pressure, shortness of breath, asthma, nasal inflammation or allergy, nasal dryness, voice changes, gastrointestinal ulcer or discomfort, constipation, dry mouth, skin redness or itching, rash, hair color change, loss of eyelashes, joint pain, muscle pain, general weakness.
Frequency not known: cannot be estimated from the available data
Eye effects:inflammation of the back of the eye, sunken eyes.
Other effects:depression, anxiety, insomnia, feeling of false movement, ringing in the ears, chest pain, irregular heartbeat, increased heart rate, worsening of asthma, diarrhea, nosebleeds, abdominal pain, nausea, vomiting, itching, abnormal hair growth, painful or involuntary urination (painful or involuntary urination), increased prostate cancer marker.
In children and adolescents, the most common side effects seen with TRAVATAN are redness of the eye and eyelash growth. Both side effects were seen more frequently in children and adolescents compared to adults.
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storing TRAVATAN
Keep this medicine out of the sight and reach of children.
Do not use TRAVATAN after the expiry date which is stated on the bottle and the carton after “EXP”. The expiry date is the last day of the month shown.
This medicine does not require any special storage conditions.
To avoid infections, you must discard the bottle 4 weeks after you first open it, and use a new bottle. Write the date you opened it on the space provided on the carton.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Contents of the pack and other information
What TRAVATAN contains
The active substance is travoprost 40 micrograms/ml.
The other ingredients are: Polyquaternium-1, hydrogenated castor oil, and polyoxyethylene 40, propylene glycol, sodium chloride, boric acid, mannitol, and purified water. Small amounts of hydrochloric acid or sodium hydroxide are added to maintain normal levels of acidity (pH levels).
Appearance and packaging
TRAVATAN is a liquid (a clear, colorless solution) that comes in a carton containing a 4 ml plastic bottle with a screw cap. Each bottle contains 2.5 ml of travoprost eye drops, and each bottle is in a pouch.
Pack sizes: 1 or 3 bottles.
Not all pack sizes may be marketed.
Marketing authorisation holder
Novartis Europharm Limited
Vista Building
Elm Park, Merrion Road
Dublin 4
Ireland
Manufacturer
Novartis Farmacéutica, S.A.
Gran Via de les Corts Catalanes, 764
08013 Barcelona
Spain
Novartis Manufacturing NV
Rijksweg 14
2870 Puurs-Sint-Amands
Belgium
Novartis Pharma GmbH
Roonstraße 25
D-90429 Nuremberg
Germany
Siegfried El Masnou, S.A.
Camil Fabra 58
El Masnou
08320 Barcelona
Spain
Novartis Pharma GmbH
Sophie-Germain-Strasse 10
90443 Nürnberg
Germany
You can get more information about this medicine by contacting the local representative of the marketing authorisation holder:
België/Belgique/Belgien Novartis Pharma N.V. Tél/Tel: +32 2 246 16 11 | Lietuva SIA Novartis Baltics Lietuvos filialas Tel: +370 5 269 16 50 |
| Luxembourg/Luxemburg Novartis Pharma N.V. Tél/Tel: +32 2 246 16 11 |
Ceská republika Novartis s.r.o. Tel: +420 225 775 111 | Magyarország Novartis Hungária Kft. Tel.: +36 1 457 65 00 |
Danmark Novartis Healthcare A/S Tlf: +45 39 16 84 00 | Malta Novartis Pharma Services Inc. Tel: +356 2122 2872 |
Deutschland Novartis Pharma GmbH Tel: +49 911 273 0 | Nederland Novartis Pharma B.V. Tel: +31 88 04 52 111 |
Eesti SIA Novartis Baltics Eesti filiaal Tel: +372 66 30 810 | Norge Novartis Norge AS Tlf: +47 23 05 20 00 |
Ελλáδα Novartis (Hellas) A.E.B.E. Τηλ: +30 210 281 17 12 | Österreich Novartis Pharma GmbH Tel: +43 1 86 6570 |
España Novartis Farmacéutica, S.A. Tel: +34 93 306 42 00 | Polska Novartis Poland Sp. z o.o. Tel.: +48 22 375 4888 |
France Novartis Pharma S.A.S. Tél: +33 1 55 47 66 00 | Portugal Novartis Farma - Produtos Farmacêuticos, S.A. Tel: +351 21 000 8600 |
Hrvatska Novartis Hrvatska d.o.o. Tel. +385 1 6274 220 | România Novartis Pharma Services Romania SRL Tel: +40 21 31299 01 |
Ireland Novartis Ireland Limited Tel: +353 1 260 12 55 | Slovenija Novartis Pharma Services Inc. Tel: +386 1 300 75 50 |
Ísland Vistor hf. Sími: +354 535 7000 | Slovenská republika Novartis Slovakia s.r.o. Tel: + 421 2 5542 5439 |
Italia Novartis Farma S.p.A. Tel: +39 02 96 54 1 | Suomi/Finland Novartis Finland Oy Puh/Tel: +358 (0)10 6133 200 |
Κúπρος Novartis Pharma Services Inc. Τηλ: +357 22 690 690 | Sverige Novartis Sverige AB Tel: +46 8 732 32 00 |
Latvija SIA Novartis Baltics Tel: +371 67 887 070 |
Date of last revision of this leaflet
Other sources of information
Detailed information on this medicine is available on the European Medicines Agency web site: http://www.ema.europa.eu
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to TRAVATAN 40 micrograms/ml eye drops, solutionDosage form: EYE DROP, 40 micrograms/mlActive substance: travoprostManufacturer: Alfred E. Tiefenbacher Gmbh & Co. KgPrescription requiredDosage form: EYEDROP, 40 micrograms/mlActive substance: travoprostManufacturer: Horus PharmaPrescription requiredDosage form: EYE DROP, 40 MICROGRAMS/MLActive substance: travoprostManufacturer: Laboratorio Stada S.L.Prescription required
Online doctors for TRAVATAN 40 micrograms/ml eye drops, solution
Discuss questions about TRAVATAN 40 micrograms/ml eye drops, solution, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions