
TRANSLARNA 1000 mg ORAL SUSPENSION GRANULES

How to use TRANSLARNA 1000 mg ORAL SUSPENSION GRANULES
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Translarna 125 mg granules for oral suspension
Translarna 250 mg granules for oral suspension
Translarna 1,000 mg granules for oral suspension
ataluren
This medicinal product is subject to additional monitoring, which will allow for the quick identification of new safety information. You can help by reporting any side effects you may get. The last section of the leaflet contains information on how to report side effects.
Read all of this leaflet carefully before you start taking this medicine, because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Translarna and what is it used for
- What you need to know before you take Translarna
- How to take Translarna
- Possible side effects
- Storage of Translarna
- Contents of the pack and other information
1. What is Translarna and what is it used for
Translarna is a medicine that contains the active substance ataluren.
Translarna is used to treat Duchenne muscular dystrophy caused by a genetic defect that affects the normal function of muscles.
Translarna is used to treat patients aged 2 years and older who can walk.
Your doctor should have carried out tests on you or your child before starting treatment with Translarna to confirm that your disease is suitable for this medicine.
How does Translarna work?
Duchenne muscular dystrophy is caused by genetic changes that produce an abnormality of a muscle protein called dystrophin, which is necessary for muscles to work properly. Translarna makes it possible to produce functional dystrophin and helps muscles to work properly.
2. What you need to know before you take Translarna
Do not take Translarna
- if you are allergic to ataluren or any of the other ingredients of this medicine (listed in section 6).
- if you are receiving antibiotics such as gentamicin, tobramycin or streptomycin by injection.
Warnings and precautions
Your doctor should have carried out a blood test to confirm that your disease is suitable for treatment with Translarna. If you have kidney problems, your doctor will carry out regular checks on your kidneys.
If you have severe kidney problems (eGFR <30 ml/min) or if you are receiving dialysis because your kidneys are not working (end-stage kidney disease), your doctor will decide if treatment with Translarna is suitable for you.
Your doctor will check the levels of lipids (fats such as cholesterol and triglycerides) in your blood and your kidney function every 6 or 12 months. If you are taking corticosteroid medicines, your doctor will check your blood pressure every 6 months.
Children and adolescents
Do not give this medicine to children under 2 years of age or weighing less than 12 kg, as it has not been tested in this group of patients.
Other medicines and Translarna
Tell your doctor if you are using, have recently used or might use any other medicines. In particular, do not take Translarna with gentamicin, tobramycin or streptomycin antibiotics given by injection. They may affect your kidney function.
You should tell your doctor if you are taking any of the following medicines:
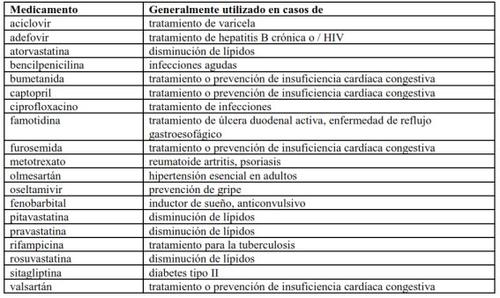
Some of these medicines have not been tested together with Translarna, and your doctor may decide to monitor you closely.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant or are planning to have a baby, ask your doctor for advice before taking this medicine. If you become pregnant while taking Translarna, tell your doctor immediately, as it is not recommended to take Translarna during pregnancy or breastfeeding.
Driving and using machines
If you feel dizzy, do not drive or use machines.
3. How to take Translarna
Follow exactly the administration instructions of this medicine given by your doctor or pharmacist. If you are unsure, ask your doctor or pharmacist again.
Translarna is available in sachets of the following concentrations: 125 mg, 250 mg and 1,000 mg of ataluren per sachet. Your doctor or pharmacist will tell you the exact number of sachets and which concentration you should take at any given time.
Your dose of Translarna depends on your body weight. The recommended dose is 10 mg/kg body weight in the morning, 10 mg/kg body weight at midday and 20 mg/kg body weight in the evening (for a total daily dose of 40 mg/kg body weight).
This medicine is taken by mouth mixed with liquid or with a semi-solid food.
Do not open the sachet until you are about to take it and use the entire contents of the sachet. All the contents of the sachet should be mixed with at least 30 ml of liquid (water, milk, fruit juice) or 3 tablespoons of semi-solid food (yogurt or apple sauce). Mix the prepared dose well before taking it. You can increase the amount of liquid or semi-solid food if you prefer.
Dosing table
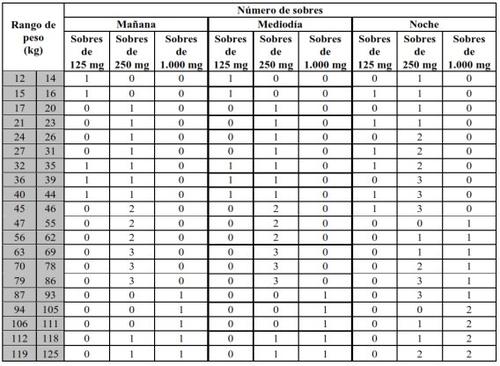
Take Translarna by mouth 3 times a day, in the morning, at midday and in the evening. There should be an interval of 6 hours between the morning dose and the midday dose, 6 hours between the midday dose and the evening dose, and 12 hours between the evening dose and the first dose of the next day. For example, you can take Translarna in the morning at 7:00 with breakfast, at midday at 13:00 with lunch and again in the evening at around 19:00 with dinner.
Drink water or other fluids regularly to avoid dehydration while taking Translarna.
If you take more Translarna than you should
If you have taken more Translarna than the recommended dose, contact your doctor.
You may experience headache, nausea, vomiting or diarrhea.
If you forget to take Translarna
If you have missed a dose and it is less than 3 hours since the usual morning or midday dose, or less than 6 hours since the evening dose, you can still take the dose. Remember to take the next dose at the usual time.
If you have missed a dose and it is more than 3 hours since the usual morning or midday dose, or more than 6 hours since the evening dose, do not take the dose. But take the next doses at the usual time.
Do not take a double dose to make up for a forgotten dose. It is important that you take the correct dose. Translarna may not be as effective in treating your symptoms if you take more than the recommended dose.
If you stop taking Translarna
Do not stop taking Translarna without talking to your doctor.
If you have any further questions on the use of this medicine, ask your doctor.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. After taking Translarna, you may experience one or more of the following side effects:
Very common side effects (may affect more than 1 in 10 people):
- Vomiting
Common side effects (may affect up to 1 in 10 people):
- Lack of appetite
- High levels of triglycerides in the blood
- Headache
- Nausea
- Weight loss
- High blood pressure
- Cough
- Nosebleeds
- Constipation
- Gas
- Stomach discomfort
- Stomach pain
- Rash
- Pain in arms or legs
- Chest pain
- Bedwetting
- Blood in the urine
- Fever
Frequency not known (cannot be estimated from the available data):
- Increased blood lipids
- Increased kidney function tests
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Translarna
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the packaging and sachet after “EXP”. The expiry date is the last day of the month stated.
No special storage conditions are required.
Take each prepared dose immediately after preparation. If the prepared dose is refrigerated (2 - 8 °C), it must be discarded if not used within 24 hours of preparation or within 3 hours if stored at room temperature (15 - 30 °C).
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
Composition of Translarna
Translarna is available in 3 concentrations, which contain 125 mg, 250 mg and 1,000 mg of the active substance ataluren, respectively. The other ingredients (excipients) are: polydextrose (E1200), macrogol, poloxamer, mannitol (E421), crospovidone, hydroxyethyl cellulose, artificial vanilla flavor, (maltodextrin, artificial flavorings and propylene glycol), colloidal silica (E551) and magnesium stearate.
Appearance and packaging of the product
Translarna is a white to off-white granule for oral suspension in sachets.
Translarna is available in packs containing 30 sachets.
Marketing authorisation holder and manufacturer
PTC Therapeutics International Limited
5th Floor
3 Grand Canal Plaza
Grand Canal Street Upper
Dublin 4
D04 EE70
Ireland
You can request more information about this medicine from the local representative of the marketing authorisation holder:
Manufacturer
Almac Pharma Services
22 Seagoe Industrial Estate
Craigavon BT63 5QD United Kingdom
PTC Therapeutics International Limited
5th Floor
3 Grand Canal Plaza
Grand Canal Street Upper
Dublin 4
D04 EE70
Ireland
Almac Pharma Services (Ireland) Limited
Finnabair Industrial Estate
Dundalk, Co. Louth, A91 P9KD
Ireland
Date of last revision of this leaflet:
This medicine has been authorised with a “conditional approval”. This type of approval means that more information on this medicine is expected.
The European Medicines Agency will review new information on this medicine at least once a year and this leaflet will be updated as necessary.
Detailed information on this medicine is available on the European Medicines Agency website: http://www.ema.europa.eu. There are also links to other websites on rare diseases and orphan medicines.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to TRANSLARNA 1000 mg ORAL SUSPENSION GRANULESDosage form: ORAL SOLUTION/SUSPENSION, 125mgActive substance: atalurenManufacturer: Ptc Therapeutics International LimitedPrescription requiredDosage form: ORAL SOLUTION/SUSPENSION, 250mgActive substance: atalurenManufacturer: Ptc Therapeutics International LimitedPrescription requiredDosage form: ORAL SOLUTION/SUSPENSION, 8.86 mg/mlActive substance: givinostatManufacturer: Italfarmaco S.P.A.Prescription required
Online doctors for TRANSLARNA 1000 mg ORAL SUSPENSION GRANULES
Discuss questions about TRANSLARNA 1000 mg ORAL SUSPENSION GRANULES, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions






