EVRYSDI 0.75 mg/ml ORAL SOLUTION POWDER


How to use EVRYSDI 0.75 mg/ml ORAL SOLUTION POWDER
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Evrysdi 0.75 mg/ml powder for oral solution
risdiplam
This medicine is subject to additional monitoring, which will allow for quick identification of new safety information. You can help by reporting any side effects you may get. The last section of this leaflet includes information on how to report side effects.
Read all of this leaflet carefully before you start taking this medicine, because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you or your child only. Do not pass it on to others, as it may harm them, even if their symptoms are the same as yours.
- If you or your child experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Evrysdi and what is it used for
- What you need to know before you or your child starts taking Evrysdi
- How to take Evrysdi
- Possible side effects
- Storage of Evrysdi
- Contents of the pack and other information
1. What is Evrysdi and what is it used for
What is Evrysdi
Evrysdi is a medicine that contains the active substance risdiplam.
What Evrysdi is used for
Evrysdi is used to treat spinal muscular atrophy (SMA), a genetic disease.
What is spinal muscular atrophy
SMA is caused by a lack of a protein called survival motor neuron (SMN) protein in the body. The lack of SMN protein can cause you or your child to lose motor neurons, which are nerve cells that control muscles. This can lead to muscle weakness and loss of muscle mass that can affect daily movements such as control of the head and neck, sitting, crawling, and walking. The muscles used for breathing and swallowing can also become weak.
How Evrysdi works
Risdiplam, the active substance in Evrysdi, works by helping the body to produce more SMN protein. This leads to fewer motor neurons being lost, which can improve muscle function in people with SMA.
In babies with SMA Type 1 treated in clinical trials for 1 year, Evrysdi has helped to:
- increase survival and reduce the need for a ventilator to help them breathe, compared to untreated babies with SMA (it is expected that only 25% of untreated babies will be alive without permanent ventilation after 14 months of age, compared to 85% of patients after 1 year of treatment with Evrysdi),
- maintain the ability to feed by mouth in 83% of patients.
In children (from infants to adolescents) and adults with SMA Type 2 and 3, Evrysdi may help to maintain or improve muscle control.
2. What you need to know before you or your child starts taking Evrysdi
Do not take Evrysdi:
- if you or your child is allergic to risdiplam or any of the other ingredients of this medicine (listed in section 6).
If you are unsure, consult your doctor or pharmacist before you or your child starts taking Evrysdi.
Warnings and precautions
Talk to your doctor, nurse, or pharmacist before you or your child starts taking Evrysdi.
Treatment with Evrysdi may harm an unborn baby or affect male fertility. See “Pregnancy, contraception, breastfeeding, and male fertility” for more information.
Other medicines and Evrysdi
Tell your doctor or pharmacist if you or your child is taking, has recently taken, or might take any other medicines.
In particular, tell your doctor, pharmacist, or nurse if you or your child is taking or has taken in the past any of the following medicines:
- metformin - a medicine used to treat type 2 diabetes
- medicines for the treatment of SMA
Pregnancy, contraception, breastfeeding, and male fertility
Pregnancy
- do not take Evrysdi if you are pregnant. This is because taking this medicine during pregnancy may harm the unborn baby.
- before starting treatment with Evrysdi, your doctor may perform a pregnancy test. This is because Evrysdi may harm the unborn baby.
- if you become pregnant while taking Evrysdi, tell your doctor immediately.
You and your doctor will decide what is best for you and the unborn baby.
Contraception
For women
do not become pregnant:
- while taking Evrysdi and
- for 1 month after stopping Evrysdi.
Talk to your doctor about reliable methods of contraception to be used during treatment and for 1 month after stopping treatment.
For men
if your partner is a woman of childbearing age, you must avoid fathering a child. Use reliable methods of contraception (e.g. condoms):
- while taking Evrysdi and
- for 4 months after stopping Evrysdi.
Talk to your healthcare professional about reliable methods of contraception to be used.
Breastfeeding
do not breastfeed while taking this medicine. This is because Evrysdi may pass into breast milk and may harm the baby.
Talk to your doctor about whether you should stop breastfeeding or stop taking Evrysdi.
Male fertility
based on findings in animals, Evrysdi may reduce male fertility during treatment and for 4 months after the last dose. If you are planning to father a child, consult your doctor for advice.
do not donate sperm during treatment and for 4 months after the last dose of Evrysdi.
Driving and using machines
it is unlikely that Evrysdi will affect your ability to drive and use machines.
Evrysdi contains sodium
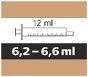
Evrysdi contains a small amount of sodium (salt) - less than 1 mmol of sodium (23 mg) per maximum daily dose of 5 mg (6.6 ml of 0.75 mg/ml oral solution). This means that it is essentially “sodium-free” and can be used by people on a low-sodium diet.
Evrysdi contains 0.375 mg of sodium benzoate per ml. Sodium benzoate may increase the risk of jaundice (yellowing of the skin and eyes) in newborn babies (up to 4 weeks of age).
Evrysdi contains isomaltose
Evrysdi contains 2.97 mg of isomaltose per ml. If your doctor has told you that you or your child has an intolerance to some sugars, consult your doctor before taking this medicine.
3. How to take Evrysdi
follow the instructions for administration of this medicine exactly as told by your doctor or pharmacist. If you are unsure, consult your doctor or pharmacist again. you must receive Evrysdi as a liquid, in a bottle. if the medicine in the bottle is a powder, do not use it and contact your pharmacist.
you must also carefully read and follow the leafletentitled “Instructions for use” on how to take or administer Evrysdi.
How much Evrysdi to take
- adolescents and adults:the daily dose of Evrysdi is 5 mg (6.6 ml of the oral solution).
- babies and children:your doctor will decide the correct dose of Evrysdi based on your child’s age and weight.
you or your child must take the daily dose as told by your doctor.do not change the dose without talking to your doctor.
when and how to take Evrysdi
- Evrysdi is a liquid that is prepared by the pharmacist and referred to in this leaflet as a “solution” or “medicine”.
- take Evrysdi once a day, approximately at the same time every day. this will help you remember when to take the medicine.
- drink water after taking the medicine. do not mix this medicine with milk or milk powder.
- take or administer Evrysdi immediately after the medicine has been drawn into the oral syringe. if it is not taken within 5 minutes, discard the medicine from the oral syringe and draw a new dose.
- if Evrysdi comes into contact with your skin or your child’s skin, wash the area with water and soap.
read the “instructions for use” leaflet
a leafletentitled “instructions for use” is included in the pack, which explains how to draw the dose using the reusable oral syringe provided. you (or your child) can take the medicine:
- by mouth,
- through a gastrostomy tube or
- through a nasogastric tube.
how long to take Evrysdi
your doctor will tell you how long you or your child should take Evrysdi. do not stop treatment with Evrysdi unless your doctor tells you to.
if you or your child takes more Evrysdi than you should
if you take more Evrysdi than you should, talk to a doctor or go to hospital immediately. take the medicine pack and this leaflet with you.
if you or your child misses a dose of Evrysdi, or vomits after taking the dose
- if it is less than 6 hours since you or your child would normally take Evrysdi, take the missed dose as soon as you remember.
- if it is more than 6 hours since you or your child would normally take Evrysdi, skip the missed dose and take the next dose at the usual time. do not take a double dose to make up for the missed dose.
- if you or your child vomits after taking a dose of Evrysdi, do not take an additional dose. instead, take the next dose at the usual time the next day.
if you spill Evrysdi
if you spill Evrysdi, wipe the area with a dry paper towel and clean with soap and water. throw the paper towel away in the trash and wash your hands well with soap and water.
4. Possible side effects
like all medicines, this medicine can cause side effects, although not everybody gets them.
very common:may affect more than 1 in 10 people
- diarrhea
- rash
- headache
- fever
common:may affect up to 1 in 10 people
- nausea
- mouth ulcers
- bladder infection
- joint pain
the following side effect has been reported since the marketing authorization of Evrysdi, but the frequency is unknown:
- inflammation of small blood vessels that mainly affects the skin (cutaneous vasculitis).
reporting of side effects
if you experience any side effects, talk to your doctor, pharmacist, or nurse. this includes any possible side effects not listed in this leaflet. you can also report side effects directly via the national reporting system listed in Appendix V. by reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Evrysdi
- keep this medicine out of the sight and reach of children.
- store the oral solution in a refrigerator (between 2°C and 8°C). if necessary, you or your caregiver can store the oral solution at room temperature (below 40°C) for a maximum of 120 hours (5 days) in total. store the oral solution back in the refrigerator when it is no longer necessary to store it at room temperature.
- monitor the total time the oral solution is out of the refrigerator (below 40°C). as stated above, the sum of the time intervals out of the refrigerator must not exceed 120 hours.
- the oral solution is stable for 64 days after preparation by the pharmacist, as long as it is stored in a refrigerator between 2°C and 8°C. the pharmacist will write the expiration date on the label of the bottle and on the original packaging in “discard after”. do not use this solution after the date stated in “discard after” or discard the medicine if the bottle has been stored at room temperature (below 40°C) for more than 120 hours (5 days) in total.
- discard the medicine if the bottle has been stored above 40°C at any time.
- store the medicine in the original bottle to protect it from light.
- store the medicine bottle upright, with the cap tightly closed.
- once the medicine has been drawn into the oral syringe, use Evrysdi immediately. do not store the Evrysdi solution in the oral syringe.
medicines should not be disposed of via wastewater or household waste. ask your pharmacist how to dispose of medicines no longer required. this will help protect the environment.
6. Contents of the pack and other information
what Evrysdi contains
- the active substance of the oral solution is risdiplam.
- each ml of the oral solution contains 0.75 mg of risdiplam.
- the other ingredients are mannitol (E 421), isomaltose (E 953), strawberry flavor, tartaric acid (E 334), sodium benzoate (E 211), macrogol/polyethylene glycol 6000, sucralose, ascorbic acid (E 300), and disodium edetate dihydrate (see section 2 “Evrysdi contains sodium” and “Evrysdi contains isomaltose”).
appearance of Evrysdi and contents of the pack
- powder for oral solution, which is provided as an oral solution after preparation by the pharmacist.
- the oral solution is yellow-green to yellow in color and has a strawberry flavor, the volume of the solution is 80 ml.
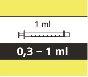
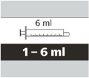
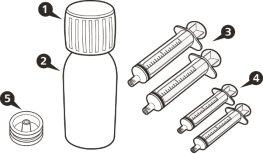
- each pack contains 1 bottle, 1 bottle adapter, and reusable amber oral syringes (2 of 1 ml, 2 of 6 ml, and 1 of 12 ml), with graduations to help draw the correct dose.
marketing authorization holder
Roche Registration GmbH
Emil-Barell-Strasse 1
79639 Grenzach-Wyhlen
Germany
manufacturer
Roche Registration AG
Emil-Barell-Strasse 1
79639 Grenzach-Wyhlen
Germany
for further information about this medicine, you can contact the local representative of the marketing authorization holder:
|
Czech Republic Roche s.r.o. Tel: +420 - 2 20382111 | Hungary Roche (Magyarország) Kft. Tel: +36 1 279 4500 |
Denmark Roche Pharmaceuticals A/S Tlf: +45 - 36 39 99 99 | Malta (see Ireland) |
Germany Roche Pharma AG Tel: +49 (0) 7624 140 | Netherlands Roche Nederland B.V. Tel: +31 (0) 348 438050 |
Estonia Roche Eesti OÜ Tel: + 372 - 6 177 380 | Norway Roche Norge AS Tlf: +47 - 22 78 90 00 |
Greece Roche (Hellas) A.E. Τηλ: +30 210 61 66 100 | Austria Roche Austria GmbH Tel: +43 (0) 1 27739 |
Spain Roche Farma S.A. Tel: +34 - 91 324 81 00 | Poland Roche Polska Sp.z o.o. Tel: +48 - 22 345 18 88 |
France Roche Tél: +33 (0) 1 47 61 40 00 | Portugal Roche Farmacêutica Química, Lda Tel: +351 - 21 425 70 00 |
Croatia Roche d.o.o. Tel: +385 1 4722 333 | Romania Roche România S.R.L. Tel: +40 21 206 47 01 |
Ireland Roche Products (Ireland) Ltd. Tel: +353 (0) 1 469 0700 | Slovenia Roche farmacevtska družba d.o.o. Tel: +386 - 1 360 26 00 |
Iceland Roche Pharmaceuticals A/S c/o Icepharma hf Sími: +354 540 8000 | Slovakia Roche Slovensko, s.r.o. Tel: +421 - 2 52638201 |
Italy Roche S.p.A. Tel: +39 - 039 2471 | Finland Roche Oy Puh/Tel: +358 (0) 10 554 500 |
Cyprus Γ.Α.Σταμ?της & Σια Λτδ. Τηλ: +357 - 22 76 62 76 | Sweden Roche AB Tel: +46 (0) 8 726 1200 |
Latvia Roche Latvija SIA Tel: +371 - 6 7039831 | United Kingdom (Northern Ireland) Roche Products (Ireland) Ltd. Tel: +44 (0) 1707 366000 |
date of last revision of this leaflet:
other sources of information
detailed information on this medicine is available on the European Medicines Agency website: https://www.ema.europa.eu.
Instructions for Use - Administration
Evrysdi 0.75mg/ml powder for oral solution
risdiplam
Make sure to read and understand these Instructions for Usebefore starting to use Evrysdi. These instructions show how to prepare and administer Evrysdi using an oral syringe, gastrostomy tube (G-tube), or nasogastric tube (NG-tube).
If you have any doubts about how to use Evrysdi, contact your doctor or pharmacist.
Evrysdi should come as a liquid in a bottle when you receive it. The pharmacist prepares the Evrysdi oral solution. If the medication in the bottle is a powder, do notuse it and contact your pharmacist.
Important Information about Evrysdi ? Ask your doctor or pharmacist to show you which oral syringe to use and how to measure the prescribed daily dose. ? Always use the reusable oral syringes provided in the packaging to measure the daily dose. ? Contact your doctor or pharmacist if the oral syringe(s) are lost or damaged. They will tell you how to continue taking the medication. ? Refer to “How to Select the Correct Oral Syringe for the Evrysdi Dose”. Ask your pharmacist if you have any doubts about how to select the correct oral syringe. ? If the bottle adapter is not on the bottle, do notuse Evrysdi and contact your pharmacist.
? Do notuse Evrysdi after the Discard afterdate on the bottle label or if you or your caregiver have kept the bottle at room temperature (below 40°C) for more than 120 hours (5 days) in total. Ask your pharmacist for the Discard afterdate if it is not written on the bottle label.
? Do notmix Evrysdi with milk or powdered milk. ? Do notuse Evrysdi if the bottle or oral syringe is damaged. ? Avoidcontact between Evrysdi and skin. If Evrysdi comes into contact with skin, wash the area with water and soap. ? If you spill Evrysdi, wipe the area with a dry paper towel and then wash it with water and soap. Throw the paper towel away and wash your hands well with water and soap. ? If there is not enough Evrysdi left in the bottle for the prescribed dose, discard the bottle with the remaining Evrysdi and the used oral syringe according to local requirements; use a new bottle of Evrysdi to get a full dose. Do not mixEvrysdi from the new bottle with the bottle you are currently using. | ||||||||
Each Box of EVRYSDI Contains (see FigureA):
|
Figure A | |||||||
Storage of Evrysdi Refer to section 5 “Storage of Evrysdi” of the package insert for complete information. | ||||||||
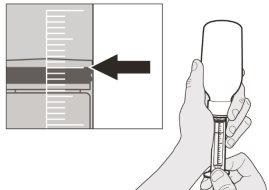
How to Select the Correct Oral Syringe for the Evrysdi Dose
| ||||||||
How to Withdraw the Evrysdi Dose | ||||||||
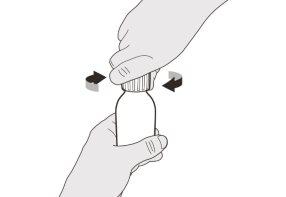
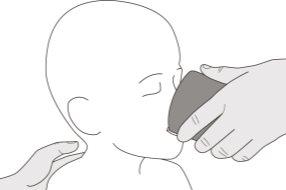
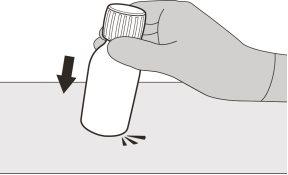
Figure B | Step A1 Remove the cap by pushing down and then twisting the cap to the left (counterclockwise) (see Figure B). Do not discard the cap. | |||||||
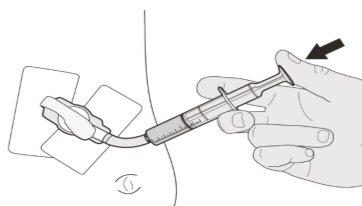
Figure C | Step A2 Push the plunger of the oral syringe down to the bottom to remove air from the oral syringe (see Figure C). | |||||||
Figure D | Step A3 Hold the bottle upright and insert the tip of the oral syringe into the bottle adapter (see Figure D). | |||||||
Figure E | Step A4 Carefully turn the bottle upside down with the tip of the oral syringe firmly inserted into the bottle adapter (see Figure E). | |||||||
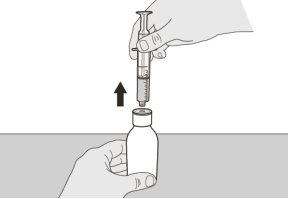
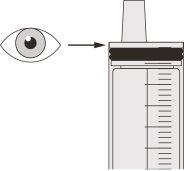
Figure F | Step A5 Slowly pull the plunger to withdraw the Evrysdi dose. The top of the black plunger should align with the ml mark on the oral syringe corresponding to the daily dose (see Figure F). Once the correct dose is withdrawn, keep the plunger in place to avoid it moving. | |||||||
Figure G | Step A6 Continue to keep the plunger in place so it does not move.Leave the oral syringe in the bottle adapter and turn the bottle upright. Place the bottle on a flat surface. Remove the oral syringe from the bottle adapter by gently pulling it upwards (see Figure G). | |||||||
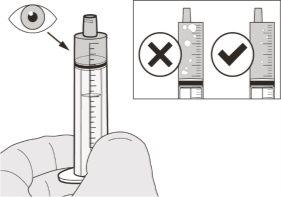
Figure H | Step A7 Hold the oral syringe with the tip facing upwards. Inspect the medication inside the oral syringe. Ifthere are large air bubbles in the oral syringe (see Figure H) or Take or administer Evrysdi immediately after withdrawing it with the oral syringe. If it is not taken within 5minutes, discard the medication from the oral syringe and withdraw a new dose. | |||||||
Figure I | Step A8 Put the cap back on the bottle. Twist the cap to the right (clockwise) to close the bottle properly (see Figure I). Do not remove the bottle adapter from the bottle. | |||||||
If you are going to take the Evrysdi dose by mouth, follow the instructions given in “B) How to Take the Evrysdi Dose by Mouth”. If you are going to take the Evrysdi dose through a gastrostomy tube, follow the instructions given in “C) How to Administer the Evrysdi Dose through a Gastrostomy Tube (G-tube)”. If you are going to take the Evrysdi dose through a nasogastric tube, follow the instructions given in “D) How to Administer the Evrysdi Dose through a Nasogastric Tube (NG-tube)”. The Evrysdi oral syringes are designed to be compatible with the ENFit® system. If the feeding tube is not compatible with ENFit®, you may need an ENFit® transition connector to connect the Evrysdi syringe to the G-tube or NG-tube. | ||||||||
| ||||||||
Stay seated and upright when taking the Evrysdi dose by mouth. | ||||||||
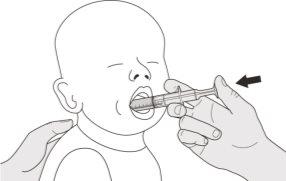
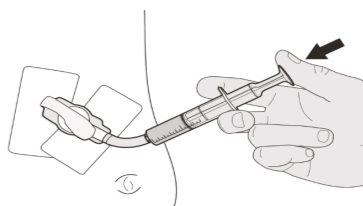
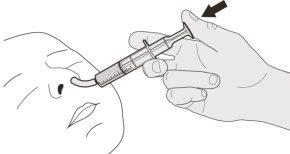
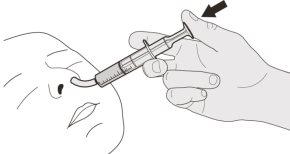
Figure J | Step B1 Place the oral syringe inside the mouth with the tip against either cheek. Slowlypush the plunger down to the bottom to administer the complete Evrysdi dose (see Figure J). Administering Evrysdi in the back of the throat or too quickly can cause choking. | |||||||
Figure K | Step B2 Check that no medication is left in the oral syringe (see Figure K). | |||||||
Figure L | Step B3 Drinka little water immediately after taking the Evrysdi dose (see Figure L). Go to StepE to clean the syringe. | |||||||
| ||||||||
If you are going to administer Evrysdi through a gastrostomy tube, ask your doctor or nurse to show you how to inspect the gastrostomy tube before administering Evrysdi. | ||||||||
Figure M | Step C1 Place the tip of the oral syringe into the gastrostomy tube. Slowly push the plunger down to the bottom to administer the complete Evrysdi dose (see Figure M). | |||||||
Figure N | Step C2 Check that no medication is left in the oral syringe (see Figure N). | |||||||
Figure O | Step C3 Flush the gastrostomy tube with 10-20 ml of water immediately after administering the Evrysdi dose (see Figure O). Go to StepE to clean the syringe. | |||||||
| ||||||||
If you are going to administer Evrysdi through a nasogastric tube, ask your doctor or nurse to show you how to inspect the nasogastric tube before administering Evrysdi. | ||||||||
Figure P | Step D1 Place the tip of the oral syringe into the nasogastric tube. Slowly push the plunger down to the bottom to administer the complete Evrysdi dose (see Figure P). | |||||||
Figure Q | Step D2 Check that no medication is left in the oral syringe (see Figure Q). | |||||||
Figure R | Step D3 Flush the nasogastric tube with 10-20 ml of water immediately after administering the Evrysdi dose (see Figure D). Go to StepE to clean the syringe. | |||||||
| ||||||||
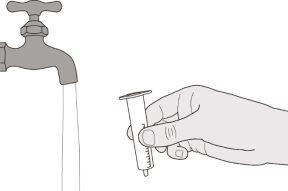
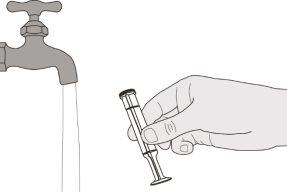
Figure S | Step E1 Remove the plunger from the oral syringe. Rinse the oral syringe body well with clean water (see Figure S). | |||||||
Figure T | Step E2 Rinse the plunger well with clean water (see Figure T). | |||||||
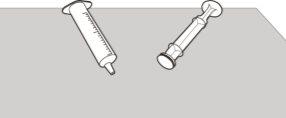
Figure U | Step E3 Check that the oral syringe body and plunger are clean. Place the oral syringe body and plunger on a clean surface, in a safe place, to dry (see Figure U). Wash your hands. Once dry, reassemble the plunger into the oral syringe body and store the syringe with the medication. |
Reconstitution Instructions
Evrysdi 0.75mg/ml
powder for oral solution
risdiplam
Reconstitution Instructions
(FOR HEALTHCARE PROFESSIONALS ONLY [E.G., PHARMACISTS])
Each Evrysdi box contains (see figureA):
|
Figure A |
Important Information about Evrysdi ? Avoid inhalingEvrysdi powder. ? Wear gloves. ?Do notuse it after the powder's expiration date has passed. The powder's expiration date is printed on the vial label. ? Do notdispense the reconstituted solution if the "Discard after" date of the solution is later than the expiration date of the original powder. ? Avoid skin contactwith the medication. If the medication comes into contact with the skin, wash the area with water and soap. ? Do notuse the medication if any of the supplies are missing or damaged. ? Use purified water or water for injectable preparations to reconstitute the medication. ? Do not add oral syringes other than those supplied in the packaging. | |
Storage of Evrysdi ? Store the powder (unreconstituted medication) at room temperature and in the packaging. ? Store the solution (reconstituted medication) in the refrigerator (between 2 °C and 8 °C) and in the packaging in a vertical position. ? Store the oral solution in the original vial and always keep the vial in a vertical position with the cap perfectly closed. | |
Reconstitution | |
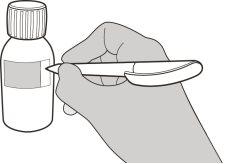
Figure B | Step1 Gently tap the bottom of the vial to loosen the powder (see figure B). |
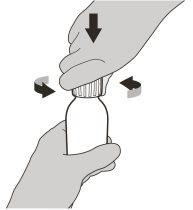
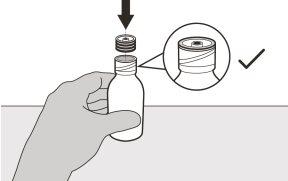
Figure C | Step2 Remove the cap by pressing down and then turning it to the left (counterclockwise) (see figure C). Do not discard the cap. |
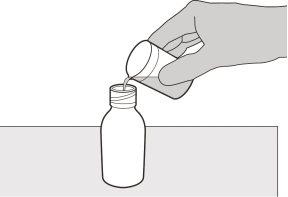
Figure D | Step3 Carefully pour 79 ml of purified water or water for injectable preparations into the medication vial (see figure D). |
Figure E | Step4 Hold the medication vial with one hand on a table. Insert the vial adapter into the opening by pushing it down with the other hand. Make sure it is fully pressed against the edge of the vial (see figure E). |
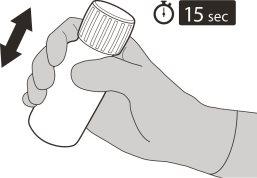
Figure F | Step5 Put the cap back on the vial. Turn the cap to the right (clockwise) to close the vial. Make sure it is perfectly closed and shake it well for 15 seconds (see figure F). Wait 10 minutes. You should have obtained a clear solution. Then, shake it well again for 15 seconds. |
Figure G | Step6 Calculate the "Discard after" date in 64daysafter reconstitution (Note: the day of reconstitution is counted as day 0. For example, if reconstitution takes place on April 1, the "Discard after" date will be June 4). Write the "Discard after" dateof the solution on the vial label (see figure G) and on the packaging. Put the vial back in its original packaging, with the syringes (in bags), the leaflet, and the Instructions for Use. Store the packaging in the refrigerator (between 2 °C and 8 °C). |
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to EVRYSDI 0.75 mg/ml ORAL SOLUTION POWDERDosage form: TABLET, 5 mgActive substance: risdiplamManufacturer: Roche Registration GmbhPrescription requiredDosage form: ORAL SOLUTION/SUSPENSION, 8.86 mg/mlActive substance: givinostatManufacturer: Italfarmaco S.P.A.Prescription requiredDosage form: INJECTABLE, 20 mgActive substance: hyaluronic acidManufacturer: Laboratorios Fidia Farmaceutica S.L.Prescription required
Online doctors for EVRYSDI 0.75 mg/ml ORAL SOLUTION POWDER
Discuss questions about EVRYSDI 0.75 mg/ml ORAL SOLUTION POWDER, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions