
TESTOSTERONE SIT 1,000 mg/4 mL Injectable Solution

How to use TESTOSTERONE SIT 1,000 mg/4 mL Injectable Solution
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Testosterona SIT 1000 mg/4ml Solution for Injection EFG
Testosterone Undecanoate
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Testosterona SIT and what is it used for
- What you need to know before you use Testosterona SIT
- How to use Testosterona SIT
- Possible side effects
- Storing Testosterona SIT
- Contents of the pack and other information
1. What is Testosterona SIT and what is it used for
Testosterona SIT contains testosterone, a male hormone, as the active ingredient.
Testosterona SIT is administered by intramuscular injection; the medicine is released over time.
Testosterone is used in adult men for testosterone replacement therapy to treat various health problems caused by low testosterone levels (male hypogonadism). This should be confirmed by two separate blood tests for testosterone and clinical symptoms such as:
- impotence
- infertility
- low sexual desire
- fatigue
- depressive mood
- bone loss caused by low hormone levels.
2. What you need to know before you use Testosterona SIT
Do not use Testosterona SIT
- if you are allergic to testosterone undecanoate or any of the other ingredients of this medicine (listed in section 6)
- if you have androgen-dependent cancer or suspect you may have prostate or breast cancer
- if you have or have had liver tumors.
Testosterone is notindicated for use in women.
Warnings and precautions
Consult your doctor before starting treatment with testosterone if you have or have ever had:
- epilepsy
- heart, liver, or kidney problems
- migraine
- interrupted breathing during sleep (sleep apnea) as it may worsen with treatment
- cancer, as calcium levels in the blood should be determined
- high blood pressure or if you are being treated for high blood pressure, as testosterone may cause an increase in blood pressure
- blood clotting problems
- bleeding disorders (e.g., hemophilia)
- thrombophilia (a blood clotting abnormality that increases the risk of blood clots in blood vessels)
- factors that increase the risk of blood clots in a vein: previous blood clots in a vein, smoking, obesity, cancer, inactivity, if a close relative has had a blood clot in a leg, lung, or other organ at a young age (e.g., below 50 years of age), or as you get older.
How to recognize a blood clot: painful swelling of a leg or sudden change in skin color, for example, becoming pale, red, or blue, sudden difficulty breathing, sudden and unexplained cough that may bring up blood, or sudden chest pain, severe dizziness or fainting, severe stomach pain, sudden loss of vision. Seek immediate medical attention if you experience any of these symptoms.
If you have severe heart, liver, or kidney failure, treatment with testosterone may cause serious complications in the form of fluid retention, which may occasionally be accompanied by congestive heart failure.
Before starting treatment and during treatment, your doctor will check the following parameters in your blood test: testosterone level and complete blood count.
If your liver is not functioning
No formal studies have been conducted in patients with liver failure. If you have ever had a liver tumor, this medicine will not be prescribed to you (see "Do not use Testosterona SIT").
Children and adolescents
Testosterone is notindicated for use in children and adolescents. There is no available information on the use of this medicine in males under 18 years of age.
Elderly patients (65 years or older)
No dose adjustment is necessary if you are over 65 years old (see "Medical examination/follow-up").
Use in athletes, bodybuilding, and doping tests
Testosterone is notindicated for increasing muscle mass in healthy individuals or for increasing physical endurance. Testosterone may produce a positive result in doping tests.
Drug abuse and dependence
Always use this medicine exactly as your doctor or pharmacist has told you.
Abuse of testosterone, especially if you take too much of this medicine alone or in combination with other anabolic androgenic steroids, can cause serious health problems to your heart and blood vessels (which can be fatal), your mental health, and/or your liver.
People who have abused testosterone may become dependent and may experience withdrawal symptoms when the dose is significantly changed or when use is suddenly stopped. Do not abuse this medicine because it can cause serious health problems, whether used alone or in combination with other anabolic androgenic steroids (see "Possible side effects").
Medical examination / follow-up
Male hormones can increase the growth of prostate cancer or increase the size of the prostate gland (benign prostatic hyperplasia). Before starting treatment with testosterone, your doctor should perform a medical examination to rule out the risk of existing prostate cancer.
Your doctor should carefully monitor your prostate and breasts, especially in elderly patients. Your doctor will also perform periodic blood tests.
Benign (non-cancerous) and malignant (cancerous) liver tumors have been reported in patients treated with hormonal products such as androgenic compounds.
Other medicines and Testosterona SIT
Tell your doctor or pharmacist if you are using or have recently used or might use any other medicines, including those obtained without a prescription. Your doctor may need to adjust the dose if you are taking other medicines, such as:
- Adrenocorticotropic hormone (ACTH) or corticosteroids (used to treat various diseases such as rheumatism, arthritis, allergic reactions, and asthma). Testosterone may increase the risk of fluid retention, especially if you have heart or liver problems.
- Medicines that make your blood more fluid (oral anticoagulants, coumarin derivatives) as they may increase the risk of bleeding. Your doctor will check your dose.
- Medicines for the treatment of diabetes. It may be necessary to adjust the dose of your antidiabetic medicine. Like other androgens, testosterone may increase the effect of insulin.
Tell your doctor if you have any blood clotting problems, as it is important for your doctor to know this information to decide if you can be treated with testosterone.
Testosterone may also affect the results of laboratory tests (e.g., thyroid gland tests). Inform your doctor or laboratory personnel that you are being treated with testosterone.
Pregnancy and breastfeeding
Testosterone is not indicated for use in women and should not be used in pregnant or breastfeeding women.
Fertility
Treatment with high doses of testosterone medicines can interrupt or reduce sperm production in a reversible manner (see also "Possible side effects").
Driving and using machines
No effects on the ability to drive and use machines have been observed.
Testosterona SIT contains benzyl benzoate
Testosterona SIT contains 2000 mg of benzyl benzoate in each ampoule/vial of 4 ml, equivalent to 500 mg/ml.
3. How to use Testosterona SIT
Your doctor will inject this medicine (1 ampoule/vial) intramuscularly, very slowly. The treatment will be repeated every 10-14 weeks, enough time to maintain testosterone levels without accumulation in the blood.
Testosterone should only be administered intramuscularly. Special care should be taken to avoid injecting the product into a blood vessel (see "Administration").
Starting treatment
Before starting treatment and during the first part of it, your doctor will determine your blood testosterone levels. Your doctor may administer a second injection, as soon as 6 weeks after the first injection, in order to quickly reach the necessary testosterone level. This will depend on your symptoms and testosterone levels.
Maintaining Testosterona SIT levels during treatment
The interval between injections should be maintained within the recommended limit of 10 to 14 weeks. Your doctor will regularly check your testosterone levels at the end of an injection interval to ensure that levels are correct. If levels are too low, your doctor may increase the frequency of injections. If levels are too high, your doctor may decrease the frequency of injections. Do not forget your treatment days, as this will not maintain your testosterone levels correctly.
If you think the effect of testosterone is too strong or too weak, tell your doctor.
If you use more Testosterona SIT than you should
Symptoms of receiving too much testosterone include:
- irritability
- nervousness
- weight gain
- frequent or prolonged erections.
Tell your doctor if you have any of the above symptoms. Your doctor will reduce the frequency of injections or stop treatment.
In case of overdose or accidental ingestion, consult your doctor, pharmacist, or call the Toxicology Information Service, phone 91 562 04 20, indicating the medicine and the amount used.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The most common adverse reactionsare acne and pain at the injection site.
Common side effects(may affect up to 1 in 10 people):
- abnormally high red blood cell counts
- weight gain
- hot flushes
- acne
- enlarged prostate and associated problems
- various reactions at the injection site (e.g., pain, bruising, or irritation)
Uncommon side effects(may affect up to 1 in 100 people):
- allergic reaction
- increased appetite, changes in some blood test results (e.g., increased sugar or fat)
- depression, emotional changes, insomnia, restlessness, aggression, or irritability
- headache, migraine, or tremors
- cardiovascular changes, high blood pressure, or dizziness
- bronchitis, sinusitis, cough, shortness of breath, wheezing, or voice changes
- diarrhea or nausea
- changes in liver test results
- hair loss or various skin reactions (e.g., itching, redness, or dry skin)
- joint or limb pain, muscle problems (e.g., spasms, pain, or stiffness), or increased creatine phosphokinase in the blood
- urinary tract changes (e.g., decreased urine flow, urinary retention, urgent need to urinate at night)
- prostate changes (e.g., prostatic dysplasia, hardening, or inflammation), changes in sexual desire, testicular pain, pain, hardening, or enlargement of the breasts, or increased levels of male and female hormones
- fatigue, general weakness, excessive sweating, or night sweats.
Rare side effects(may affect up to 1 in 1000 people):
- The oily liquid of testosterone may reach the lungs (pulmonary microembolism of oily solutions) and in rare cases may cause signs and symptoms such as cough, shortness of breath, general discomfort, excessive sweating, chest pain, dizziness, or fainting. These reactions may occur during or immediately after injection and are reversible.
Anaphylactic reactions have been reported after injection of testosterone.
In addition to the above effects, the following side effects have been observed after treatment with testosterone products: nervousness, hostility, brief interruptions of breathing during sleep (sleep apnea), skin reactions such as dandruff and seborrhea, excessive hair growth, more frequent erections, and in very rare cases, yellowing of the skin and eyes (jaundice).
Treatment with high doses of testosterone generally interrupts or reduces sperm production, although it returns to normal after stopping treatment. Testosterone replacement therapy in cases of low testicular function (hypogonadism) may rarely cause persistent and painful erections (priapism). Long-term or high-dose testosterone treatment may occasionally cause increased fluid retention and edema (swelling due to fluid retention).
In general, with testosterone-based medicines, a frequent risk of increased red blood cell count, hematocrit (percentage of red blood cells in the blood), and hemoglobin (the component of red blood cells that carries oxygen) has been observed, identified through periodic blood tests.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist, even if it is possible side effects not listed in this leaflet. You can also report them directly through the Spanish Medicines Agency's website: www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storing Testosterona SIT
Keep this medicine out of the sight and reach of children.
This medicine does not require any special storage conditions.
Do notuse this medicine after the expiry date which is stated on the carton after EXP. The expiry date is the last day of the month shown.
Medicines should notbe disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Container Contents and Additional Information
Composition of Testosterona SIT
The active ingredient is testosterone undecanoate 250 mg/ml (corresponding to 157.9 mg of testosterone). One ampoule/vial contains 1000 mg of testosterone undecanoate (corresponding to 631.5 mg of testosterone).
The other components are: benzyl benzoate and refined castor oil.
Appearance of the Product and Container Contents
Testosterona SIT is an oily solution, slightly yellow to yellow in color.
Testosterona SIT is presented in a brown glass ampoule or a brown glass vial with a rubber stopper and an aluminum cap with a plastic flip-off closure.
The contents of the containers are:
1 brown glass ampoule/brown glass vial with 4 ml of injectable solution.
Only some package sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder
Laboratorio Farmaceutico S.I.T. S.r.l.
Via Cavour, 70
27035 Mede (PV)
Italy
Manufacturer
EVER Pharma Jena GmbH
Brüsseler Str 18
07747 Jena
Germany
For further information on this medicinal product, please contact the local representative of the Marketing Authorization Holder:
Desma Laboratorio Farmacéutico SL
Paseo de la Castellana 121, Escalera Izquierda 3ºB
28046 Madrid
Telephone: +34 91 3238792
This medicinal product is authorized in the Member States of the European Economic Area and in the United Kingdom (Northern Ireland) under the following names:
- Netherlands: Testosteron undecanoaat SIT 1000 mg/4 ml, oplossing voor injectie
- Italy: Testosterone undecanoato SIT 1000 mg/4 ml, soluzione iniettabile
Date of the last revision of this leaflet:May 2024
------------------------------------------------------------------------------------------------------------------------
This information is intended only for healthcare professionals:
At cold storage temperatures, the properties of this oily solution may change temporarily (e.g., increased viscosity, turbidity). If stored at cold temperatures, the product should be brought to room temperature or body temperature before use.
The intramuscular injectable solution should be inspected visually before use; only clear and particle-free solutions should be used.
The contents of an ampoule/vial should be injected intramuscularly immediately after opening the ampoule/vial.
The medicinal product is for single use; any unused solution should be discarded.
Administration
Caution is necessary to avoid injecting the medicinal product into a blood vessel.
Like all oily solutions, this medicinal product should be injected only intramuscularly and very slowly. Pulmonary microembolism related to oily solutions may rarely cause signs and symptoms such as cough, dyspnea, discomfort, hyperhidrosis, chest pain, dizziness, paresthesia, or syncope. These reactions may occur during or immediately after injection and are reversible. Treatment is usually supportive, e.g., administration of supplemental oxygen.
Suspected anaphylactic reactions have been reported after administration of this medicinal product.
Warnings
A careful regular check of the prostate and breasts should be performed according to established methods (digital rectal examination and determination of PSA in serum) in patients receiving testosterone treatment, at least once a year and twice a year in elderly and at-risk patients (those with clinical or family factors).
In addition to laboratory tests to determine the concentration of testosterone in the blood in patients undergoing long-term androgen therapy, the following laboratory tests should be performed periodically: hemoglobin, hematocrit, liver function tests, and lipid profile.
In patients with severe heart, liver, or kidney failure or with ischemic heart disease, testosterone treatment may cause serious complications, characterized by edema with or without congestive heart failure. In this case, treatment should be discontinued immediately.
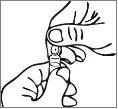
Handling Instructions for the Testosterona SIT Ampoule with "One Point Cut" (UPC) Opening System
The ampoule has a mark below the colored point. Before opening, ensure that no solution remains in the top part of the ampoule. Use both hands to open it; while holding the lower part of the ampoule with one hand, use the other hand to press outward and break the top part of the ampoule in the direction opposite the colored point.
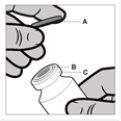
Handling Instructions for the Vial:
The vial is for single use. The contents of the vial should be injected intramuscularly immediately after loading into the syringe. After removing the plastic cover (A), do not remove the metal ring (B) or the edge cover (C).
- Country of registration
- Average pharmacy price76.17 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to TESTOSTERONE SIT 1,000 mg/4 mL Injectable SolutionDosage form: GEL, 2% testosteroneActive substance: testosteroneManufacturer: Advanz Pharma LimitedPrescription requiredDosage form: INJECTABLE, 1000 mg/dose (250 mg/ml)Active substance: testosteroneManufacturer: Grünenthal Pharma S.A.Prescription requiredDosage form: GEL, 23 mg/gActive substance: testosteroneManufacturer: The Simple Pharma Company LimitedPrescription required
Online doctors for TESTOSTERONE SIT 1,000 mg/4 mL Injectable Solution
Discuss questions about TESTOSTERONE SIT 1,000 mg/4 mL Injectable Solution, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions












