
TERBASMIN TURBUHALER 500 micrograms/inhalation INHALATION POWDER

How to use TERBASMIN TURBUHALER 500 micrograms/inhalation INHALATION POWDER
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
- Introduction
- What is Terbasmin Turbuhaler 500 micrograms/inhalation powder for inhalation and what is it used for
- What you need to know before you start using Terbasmin Turbuhaler 500 micrograms/inhalation powder for inhalation
- How to use Terbasmin Turbuhaler 500 micrograms/inhalation powder for inhalation
- Possible side effects
- Storage of Terbasmin Turbuhaler 500 micrograms/inhalation powder for inhalation
- Package contents and further information
Introduction
Package Leaflet: Information for the User
Terbasmin Turbuhaler 500 micrograms/inhalation powder for inhalation
(terbutaline sulfate)
Read all of this leaflet carefully before you start using this medicine.
|
Contents of the package leaflet
- What is Terbasmin Turbuhaler 500 micrograms/inhalation powder for inhalation and what is it used for
- What you need to know before you start using Terbasmin Turbuhaler 500 micrograms/inhalation powder for inhalation
- How to use Terbasmin Turbuhaler 500 micrograms/inhalation powder for inhalation
- Possible side effects
- Storage of Terbasmin Turbuhaler 500 micrograms/inhalation powder for inhalation
- Package contents and further information
1. What is Terbasmin Turbuhaler 500 micrograms/inhalation powder for inhalation and what is it used for
Terbasmin Turbuhaler contains the active substance terbutaline. Terbutaline belongs to a group of medicines called bronchodilators and works by widening the airways and making it easier to breathe. It is used for asthma, chronic bronchitis, emphysema, and other respiratory conditions that cause bronchoconstriction (contraction of the bronchi). When inhaled through the mouthpiece of the inhaler, the medicine reaches the lungs.
2. What you need to know before you start using Terbasmin Turbuhaler 500 micrograms/inhalation powder for inhalation
Do not use Terbasmin Turbuhaler 500 micrograms/inhalation powder for inhalation:
If you are allergic to the active substance or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions:
- If you have previously experienced any unusual reaction to other medicines, inform your doctor.
- In certain cases, Terbasmin Turbuhaler should be used with caution. You should always inform your doctor of any other conditions you may have, especially if you have a history of heart disease, irregular heartbeat, angina, diabetes, or thyroid problems (hyperthyroidism).
- This medicine has been prescribed for you to treat the disease you are suffering from. Do not use it for another condition unless your doctor tells you to. Do not give it to another person.
- There is a small risk of accumulation of part of the medicine in the mouthpiece towards the end of the inhaler's life, so it is advisable to rinse your mouth with water after each inhalation, if possible.
Using Terbasmin Turbuhaler 500 micrograms/inhalation powder for inhalation with other medicines
Tell your doctor or pharmacist if you are using or have recently used any other medicines, including those obtained without a prescription. Some medicines may alter the effect of Terbasmin Turbuhaler if used together, for example:
- Certain medicines called beta-blockers, used to lower blood pressure, for heart conditions or some eye drops.
- The combined use of Terbasmin Turbuhaler and some medicines may produce effects on the salt balance (decrease in potassium levels) in the blood. This effect is usually not important, but in certain cases, it may affect the heartbeat.
Pregnancy, breastfeeding, and fertility
Consult your doctor or pharmacist before using any medicine.
Tell your doctor before using this medicine if you are pregnant, plan to become pregnant, or are breastfeeding. You should always be cautious when using medicines during pregnancy or breastfeeding. No harmful effects have been observed in pregnant or breastfeeding women treated with this medicine. However, inform your doctor as soon as possible if you become pregnant during treatment with Terbasmin Turbuhaler.
Use in athletes:
Athletes are informed that this medicine contains a component that may produce a positive result in doping tests.
Driving and using machines
Terbasmin Turbuhaler does not affect the ability to drive and use machines.
Terbasmin Turbuhaler contains lactose
This medicine contains lactose, which is a type of sugar. If your doctor has told you that you have an intolerance to certain sugars, consult with them before using this medicine. The amount of lactose in this medicine usually does not cause problems in people with lactose intolerance.
Lactose may contain small amounts of residual milk proteins. It can cause allergic reactions in patients with milk protein allergy.
3. How to use Terbasmin Turbuhaler 500 micrograms/inhalation powder for inhalation
Follow exactly the instructions for administration of this medicine indicated by your doctor. If in doubt, consult your doctor or pharmacist again. Remember to use your medicine.
Method of use and administration route:
This medicine is administered by inhalation.
Terbasmin Turbuhaler should be used preferably when needed, rather than regularly.
Before starting to use Terbasmin Turbuhaler for the first time, it is important that you read the information included in the section "How to use Terbasmin Turbuhaler 500 micrograms/inhalation powder for inhalation" and follow the instructions carefully.
Children may not use the dosing system correctly. Therefore, it is essential to ensure that the child can follow the instructions for use correctly.
Terbasmin Turbuhaler should be used preferably when needed, rather than regularly.
Seek medical attention immediately if your asthma symptoms (cough, difficulty breathing, wheezing, or chest tightness) worsen or if you are short of breath to the point where you cannot speak, eat, or sleep.
Contact your doctor as soon as possible if you need to use Terbasmin Turbuhaler more frequently than usual to relieve your breathing problems. You may need additional medication to control your disease. Do not increase the dose of Terbasmin Turbuhaler without consulting your doctor first.
If you use Terbasmin Turbuhaler more than twice a week to treat your asthma symptoms, this indicates poorly controlled asthma and may increase the risk of severe asthma attacks (asthma exacerbations) that can have serious complications and can be life-threatening. You should consult your doctor as soon as possible to review your asthma treatment.
If you use a medicine daily to reduce inflammation in the lungs, such as "inhaled corticosteroids," it is essential that you continue to use it regularly, even if you feel better.
Dosage, administration frequency, and treatment duration:
The dose of Terbasmin Turbuhaler 500 micrograms should be individualized. Your doctor will indicate the duration of your treatment with Terbasmin Turbuhaler. Do not take more doses than your doctor has indicated.
The recommended doses of Terbasmin Turbuhaler are as follows:
Usual dose for adults and children over 12 years:1 inhalation (500 micrograms) as needed. If necessary, the dose can be increased up to 3 inhalations (1500 micrograms) in a single dose. The total dose should not exceed 12 inhalations (6000 micrograms) in 24 hours.
Usual dose for children from 3 to 12 years:1 inhalation (500 micrograms) as needed. If necessary, the dose can be increased up to 2 inhalations (1000 micrograms) in a single dose. The total dose should not exceed 8 inhalations (4000 micrograms) in 24 hours.
The effect of Terbasmin Turbuhaler is usually felt within 5 minutes after inhalation, and its duration is up to 6 hours. Treatment with Terbasmin Turbuhaler is effective even in an acute asthma attack.
If you use more Terbasmin Turbuhaler than you should
Only use the number of inhalations that your doctor has prescribed, as using more inhalations will increase the risk of side effects without improving efficacy.
The most common symptoms and signs that may occur after an overdose are:
- Nausea
- Headache
- Fast or irregular heartbeat
- Muscle cramps
- Nervousness, restlessness, or tremors
- Dizziness or unusual drowsiness
Contact your doctor if any of these symptoms concern you.
If you have used more Terbasmin Turbuhaler than you should, consult your doctor, pharmacist, or call the Toxicology Information Service, telephone 91 562 04 20, indicating the medicine and the amount used. It is recommended to take the packaging and the package leaflet of the medicine to the healthcare professional.
If you forget to use Terbasmin Turbuhaler 500 micrograms/inhalation powder for inhalation:
Do not use a double dose to make up for forgotten doses.
Terbasmin Turbuhaler should be used preferably when you need it, rather than regularly.
However, if your doctor has prescribed a regular treatment and you forget to use one of the doses, take the recommended inhalations as soon as you remember. If it is close to the time for the next inhalation, it is preferable to wait until that time.
If you have any further questions about the use of this product, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Side effects are usually not observed during the use of Terbasmin Turbuhaler. If they occur, they are usually mild and disappear on their own within one or two weeks of treatment. However, inform your doctor of the following side effects that bother you or do not disappear.
Very common side effects (may affect more than 1 in 10 patients):
- Tremors
- Headache
Common side effects (may affect up to 1 in 10 patients):
- Fast heartbeat
- Low potassium levels in the blood
- Muscle cramps
Frequency unknown*:
- Irregular heartbeat
- Nausea (feeling of discomfort)
- Agitation, restlessness, or difficulty sleeping
- Allergic reactions such as itching and rash
- Bronchospasm (contraction of the muscles of the airways, which causes "wheezing")**
* The side effects observed are from post-marketing reports, so the frequency is unknown.
** Inhaled medicines may cause bronchospasm.
Although it is not known exactly how often it occurs, some people may occasionally experience chest pain (due to heart problems such as angina). If you experience these symptoms while using Terbasmin Turbuhaler, inform your doctor, but do not stop using it unless your doctor tells you to.
If you think any of the side effects you are experiencing are serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Pharmacovigilance System for Human Use Medicines: www.notificaRAM.es.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Terbasmin Turbuhaler 500 micrograms/inhalation powder for inhalation
Keep this medicine out of the sight and reach of children.
Always replace the cap after using Terbasmin Turbuhaler.
Do not store above 30°C.
Do not use this medicine after the expiry date which is stated on the packaging and on the inhaler label after CAD/EXP. The expiry date is the last day of the month stated.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Package contents and further information
Composition of Terbasmin Turbuhaler 500 micrograms/inhalation powder for inhalation
Each dose (inhalation) contains:
- Active substance: 500 micrograms of terbutaline sulfate.
- The excipient is lactose.
Appearance of the product and package contents
Terbasmin Turbuhaler is presented as a powder for inhalation. The powder contains only terbutaline.
500 micrograms/dose:Each inhaler contains 120 doses.
Marketing Authorization Holder and Manufacturer:
The holder is:
Laboratorio Lailan, S.A.
C/ Puerto de Somport 21-23
28050 Madrid
Spain
The manufacturer is:
AstraZeneca AB
SE-151 36 Södertälje
Sweden
Instructions
How to prepare your new Terbasmin Turbuhaler inhaler
Before using your newTerbasmin Turbuhaler inhaler for the first time, you must prepare the inhaler for use as follows:
- Unscrew and lift the cap. You may hear a rattling sound.
- Hold your Terbasmin Turbuhaler upright with the blue screw at the bottom.
- Turn the blue screw as far as it will go in one direction. Then turn it as far as it will go in the other direction (it does not matter which direction you turn it first).
- You should hear a click.
- Repeat this procedure once more, turning the blue screw in both directions.
- Your Terbasmin Turbuhaler inhaler is now ready for use.
How to take an inhalation
Each time you need to take an inhalation, follow the instructions below:
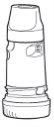
1.- Unscrew and lift the white cap. You may hear a rattling sound.
2.- Hold the inhaler upright with the blue screw at the bottom.
3.- Do not hold the mouthpiece while loading the Turbuhaler. To load the Turbuhaler with a dose, turn the blue screw as far as it will go in one direction and then turn it as far as it will go in the other direction (it does not matter which direction first). You should hear a characteristic “click”. At this moment, the Turbuhaler is loaded and ready for use. Load the Turbuhaler only when you need to use it.
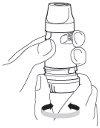
4.- Hold the inhaler away from your mouth. Breathe out gently (as much as you can without feeling uncomfortable). Do not breathe out through the Turbuhaler.
5.- Place the mouthpiece gently between your teeth, close your lips, and inhale through your mouth as forcefully and deeply as possible. Do not bite or press the mouthpiece with your teeth.
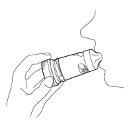
6.- Remove the inhaler from your mouth and then breathe out gently. The amount of medication released is very small, which means you may not notice its taste after inhalation. However, if you have followed the instructions for use, you can be sure that you have inhaled the dose and that the medication has reached your lungs.
7.- If you need to take a second inhalation, repeat steps 2 to 6.
8.- Replace the cap firmly after use.
9.- Rinse your mouth with water each time you inhale your dose, if possible, to remove any medication that remains in your mouth.
As with all inhalers, caregivers should ensure that children who have been prescribed Terbasmin Turbuhaler perform the inhalation technique correctly, as described above.
Do not attempt to separate or twist the mouthpiece; it is fixed to the Turbuhaler and should not be separated. The mouthpiece can be twisted, but do not twist it unnecessarily. Do not use the Turbuhaler if it is damaged or if the mouthpiece has come off the Turbuhaler.
Cleaning the Turbuhaler
Clean the outside of the mouthpiece once a week with a dry cloth; do not use water or liquids.
When to start a new Turbuhaler
The dose indicator shows how many doses are left in the Turbuhaler, starting at 120 doses when it is full.
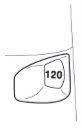
The dose indicator is marked in intervals of 10 doses, so not all doses are shown. When you first see a red mark at the end of the indicator window, there are approximately 20 doses left. During the last 10 doses, the bottom of the dose indicator is red. When the “0” at the bottom of the red indicator window reaches the center of this window, you should start a new Turbuhaler.
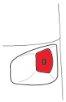
Note:
- The screw can continue to turn and make a “click”even though the Turbuhaler is empty.
- The sound you hear when shaking the inhaler is produced by a desiccant and not by the medication, so it does not indicate the amount of medication left in the Turbuhaler.
- If you load your Terbasmin Turbuhaler inhaler more than once by mistake before taking your dose, you will only inhale one dose. However, the dose indicator will record all loaded doses.
Date of the last revision of this prospectus: December 2022
Detailed and updated information on this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Average pharmacy price6.93 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to TERBASMIN TURBUHALER 500 micrograms/inhalation INHALATION POWDERDosage form: PULMONARY INHALATION, 0.294 mg salmeterol xinafoateActive substance: salmeterolManufacturer: Glaxosmithkline S.A.Prescription requiredDosage form: PULMONARY INHALATION, salmeterol 50 mcgActive substance: salmeterolManufacturer: Glaxosmithkline S.A.Prescription requiredDosage form: PULMONARY INHALATION, 25 µg salmeterol xinafoate/applicationActive substance: salmeterolManufacturer: Glaxosmithkline S.A.Prescription required
Online doctors for TERBASMIN TURBUHALER 500 micrograms/inhalation INHALATION POWDER
Discuss questions about TERBASMIN TURBUHALER 500 micrograms/inhalation INHALATION POWDER, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















