JUANOLGAR 0.51 mg/puff ORAL SPRAY SOLUTION

How to use JUANOLGAR 0.51 mg/puff ORAL SPRAY SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Leaflet: information for the user
Juanolgar 0.51 mg/puff oral spray solution
bencidamine, hydrochloride
Read this leaflet carefully before starting to use this medicine because it contains important information for you.
Follow exactly the administration instructions of the medicine contained in this leaflet or as indicated by your doctor or pharmacist.
- Keep this leaflet, as you may need to read it again.
- If you need advice or more information, consult your pharmacist.
- If you experience side effects, consult your doctor or pharmacist, even if they are side effects not listed in this leaflet. See section 4.
- You should consult a doctor if it worsens or does not improve after 2 days.
Contents of the leaflet
- What is Juanolgar and what is it used for
- What you need to know before taking Juanolgar
- How to use Juanolgar
- Possible side effects
- Storage of Juanolgar
- Package contents and additional information
1. What is Juanolgar and what is it used for
The active ingredient of this medicine is bencidamine hydrochloride. It is a topical anti-inflammatory for oral use.
Juanolgar is indicated in adults and adolescents over 12 years old for the local symptomatic relief of throat and mouth irritations that occur with pain and without fever.
2. What you need to know before taking Juanolgar
Do not use Juanolgar
- If you are allergic to bencidamine or any of the other components of this medicine (listed in section 6).
Warnings and precautions
- Consult your doctor or pharmacist before starting to use Juanolgar.
- If you have an allergy to salicylic acid and/or other analgesics or anti-inflammatory drugs (asthmatic crises, etc.), the use of Juanolgar is not recommended.
- In patients who have suffered or suffer from bronchial asthma, it can cause bronchospasm (sudden feeling of suffocation), so these patients should be cautious.
Children:
It is not recommended for use in children under 12 years old, unless advised by a doctor.
Using Juanolgar with other medicines
Tell your doctor or pharmacist if you are using, have recently used, or may need to use any other medicine.
Using Juanolgar with food, drinks, and alcohol
Do not eat or drink until 1 hour after using the medicine.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, if you think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before taking this medicine.
Do not use Juanolgar during pregnancy unless clearly necessary and advised by your doctor. If treatment is needed, use the lowest dose for the shortest possible time.
Driving and using machines
Topical use of bencidamine, at the indicated doses, does not alter the ability to drive or use other machines.
Juanolgar contains alcohol (ethanol), macrogolglycerol hydroxystearate, methyl parahydroxybenzoate, and peppermint flavor.
This medicine contains 13.6 mg of alcohol (ethanol) per puff. The amount in volume of this medicine is equivalent to less than 0.34 ml of beer or 0.14 ml of wine. The small amount of alcohol in this medicine does not produce any noticeable effect.
This medicine contains macrogolglycerol hydroxystearate, which can cause stomach upset and diarrhea.
This medicine contains methyl parahydroxybenzoate, which can cause allergic reactions (possibly delayed).
This medicine contains fragrances (peppermint flavor) with benzyl alcohol, citronellol, d-limonene, eugenol, geraniol, and linalool. Benzyl alcohol, citronellol, d-limonene, eugenol, geraniol, and linalool can cause allergic reactions.
This medicine contains less than 1 mmol of sodium (23 mg) per puff; i.e., it is essentially "sodium-free."
3. How to use Juanolgar
Follow exactly the administration instructions of this medicine as indicated by your doctor or pharmacist. In case of doubt, consult your doctor or pharmacist again.
The recommended dose is:
Adults and adolescents over 12 years old:
Apply 1 to 3 puffs per day for 4-6 days. Do not eat or drink until 1 hour after using the medicine.
In a limited number of patients, buco-pharyngeal ulcers are often a sign of serious conditions. Therefore, patients whose symptoms do not disappear within 2 days, worsen, or develop a fever should consult a doctor or dentist.
Treatment should not exceed 6 days.
It is not recommended for use in children under 12 years old, unless advised by a doctor.
This medicine is used via the buco-pharyngeal route.
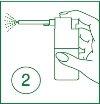
| 1 | Raise the spray nozzle. |
| 2 | Insert the nozzle into the mouth and direct the spray towards the painful area. Press the finger on the striped area of the actuator. |
On first use of the product, press the actuator several times in the air until a regular spray is obtained.
If you use more Juanolgar than you should
Due to its topical use, it is unlikely to produce poisoning. Nevertheless, if you use more Juanolgar than recommended, although very rarely, you may feel: excitement, convulsions, sweating, alterations in gait, tremors, and vomiting in children.
In case of overdose or accidental ingestion of large amounts of medicine, consult your doctor or pharmacist immediately for advice. You can also call the Toxicology Information Service, phone 91 562 04 20, indicating the medicine and the amount ingested.
If you forget to use Juanolgar
Do not use a double dose to make up for forgotten doses.
If you have any other doubts about the use of this product, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, Juanolgar can cause side effects, although not everybody gets them.
- Uncommon (at least 1 in 1,000 patients): Photosensitivity (sensitivity to sunlight).
- Rare (at least 1 in 10,000 patients): Itching, dry mouth.
- Very rare (less than 1 in 10,000 patients): Laryngospasm (contraction of the laryngeal muscles with a feeling of suffocation) and swelling.
- Not known (frequency cannot be estimated from available data):
Allergic reaction (hypersensitivity).
Severe allergic reaction (anaphylactic shock), whose signs may include difficulty breathing, pain or pressure in the chest and/or feeling of dizziness/fainting, intense itching of the skin or hives, swelling of the face, lips, tongue, and/or throat, and which can be potentially fatal.
If you experience side effects, consult your doctor or pharmacist, even if they are side effects not listed in this leaflet.
Reporting side effects
If you experience any type of side effect, consult your doctor or pharmacist, even if it is a possible side effect not listed in this leaflet. You can also report them directly through the Spanish Medicines and Healthcare Products Agency (AEMPS) website: https://www.notificaram.es
By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Juanolgar
No special storage conditions are required.
Keep this medicine out of sight and reach of children.
Do not use this medicine after the expiration date stated on the packaging, after EXP. The expiration date is the last day of the month indicated.
Medicines should not be disposed of via wastewater or household waste. Deposit the packaging and medicines you no longer need at the pharmacy's SIGRE point. If in doubt, ask your pharmacist how to dispose of the packaging and medicines you no longer need. This will help protect the environment.
6. Package contents and additional information
Juanolgar composition
The active ingredient is bencidamine hydrochloride. Each 0.17 ml puff contains 0.51 mg of bencidamine hydrochloride.
The other ingredients are: ethanol 96%, glycerol, macrogolglycerol hydroxystearate 40, peppermint flavor (contains: benzyl alcohol, citronellol, d-limonene, eugenol, geraniol, and linalool), methyl parahydroxybenzoate (E-218), sodium saccharin, and purified water.
Appearance of the product and package contents
It is presented as a solution for oral spray.
The solution is clear and colorless and is presented in bottles containing 15 ml of solution.
Marketing authorization holder and manufacturer
Marketing authorization holder
ANGELINI PHARMA ESPAÑA, S.L.
C/ Antonio Machado, 78-80.
3rd floor, module A-Australia Building
08840 Viladecans, Barcelona,
Spain
Manufacturer
A.C.R.A.F. S.p.A.,
Via Vecchia del Pinocchio, 22
60131 – Ancona,
Italy
Date of last revision of this leaflet:September 2025
Detailed information on this medicine is available on the website of the Spanish Agency for Medicines and Healthcare Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to JUANOLGAR 0.51 mg/puff ORAL SPRAY SOLUTIONDosage form: GEL/PASTE/ORAL LIQUID, 1.5 mg/mlActive substance: benzydamineManufacturer: Aristo Pharma GmbhPrescription not requiredDosage form: BUCCAL/SUCKING TABLET, 3 mgActive substance: benzydamineManufacturer: Laboratorio Reig Jofre, S.A.Prescription not requiredDosage form: BUCCAL/SUCKING TABLET, 3 mgActive substance: benzydamineManufacturer: Laboratorio Reig Jofre, S.A.Prescription not required
Online doctors for JUANOLGAR 0.51 mg/puff ORAL SPRAY SOLUTION
Discuss questions about JUANOLGAR 0.51 mg/puff ORAL SPRAY SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions