
TALIDOMIDE ACCORD 50 mg HARD CAPSULES

How to use TALIDOMIDE ACCORD 50 mg HARD CAPSULES
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Talidomida Accord 50 mg Hard Capsules EFG
WARNING
Talidomida causes birth defects and fetal death. Do not take talidomida if you are or may become pregnant. You must follow your doctor's advice on contraception
Read the entire package leaflet carefully before starting to take this medication, as it contains important information for you.
- Keep this package leaflet, as you may need to read it again.
- If you have any questions, consult your doctor, pharmacist, or nurse.
- This medication has been prescribed to you only, and you should not give it to others, even if they have the same symptoms as you, as it may harm them.
- If you experience side effects, consult your doctor, pharmacist, or nurse, even if they are not listed in this package leaflet. See section 4.
Contents of the Package Leaflet
- What is Talidomida Accord and what is it used for
- What you need to know before taking Talidomida Accord
- How to take Talidomida Accord
- Possible side effects
- Storage of Talidomida Accord
- Contents of the pack and further information
1. What is Talidomida Accord and what is it used for
What is Talidomida Accord
Talidomida Accord contains an active substance called talidomida, which belongs to a group of medicines that affect how your immune system works.
What is Talidomida Accord used for
Talidomida is used with two other medicines called "melfalan" and "prednisona" to treat adults with a type of cancer called multiple myeloma. It is used in people 65 years or older who have been recently diagnosed and have not previously used any medication for multiple myeloma, or in people under 65 years who cannot be treated with high-dose chemotherapy, as it may be difficult for the body to tolerate.
What is multiple myeloma
Multiple myeloma is a type of cancer that affects a type of white blood cell called plasma cells. These cells are produced in the bone marrow and divide uncontrollably. This can damage the bones and kidneys. Multiple myeloma usually has no cure. However, the signs and symptoms can decrease significantly or disappear for a period of time. This is called "remission".
How Talidomida Accord works
Talidomida works by helping the body's immune system and directly attacking the cancer. It works in different ways:
- It slows down the development of cancer cells.
- It slows down the growth of blood vessels in the cancer.
- It stimulates part of the immune system to attack cancer cells.
.
2. What you need to know before taking Talidomida Accord
You must receive specific instructions from your doctor, particularly about the effects of talidomida on the fetus (which are explained in the Talidomida Accord Pregnancy Prevention Program).
You must receive a patient information leaflet from your doctor. You must read it carefully and follow the instructions.
If you do not fully understand these instructions, ask your doctor to explain them again before taking talidomida. You can find more information in this section in the "Warnings and precautions" and "Pregnancy and breastfeeding" sections.
Do not take Talidomida Accord
- If you are pregnant, think you may be pregnant, or plan to become pregnant, as talidomida causes birth defects and fetal death.
- If you can become pregnant unless you are able to follow or comply with the contraceptive measures required to avoid becoming pregnant (see section 2 "Warnings and precautions" and "Pregnancy and breastfeeding").
- If you can become pregnant, your doctor will register with each prescription that the necessary measures have been taken and will provide you with this confirmation.
- If you are allergic to talidomida or any of the other components of this medication listed in section 6 "Contents of the pack and further information".
Do not take talidomida if any of the above points apply to you. If you are unsure, consult your doctor or pharmacist before starting to take talidomida.
Warnings and precautions
Consult your doctor, pharmacist, or nurse before starting to take this medication in the following cases:
For womentaking talidomida
Before starting treatment, you must consult your doctor if there is a possibility that you may become pregnant, even if you think it is unlikely. Even if you do not have menstrual bleeding after cancer treatment, you can still become pregnant.
If you can become pregnant:
- Your doctor will ensure that you have pregnancy tests:
- Before treatment.
- Every 4 weeks during treatment.
- 4 weeks after finishing treatment.
- You must use an effective method of contraception:
- For at least 4 weeks before starting treatment.
- During treatment.
- Until at least 4 weeks after finishing treatment.
Your doctor will indicate which contraceptive method you should use.
If you can become pregnant, your doctor will register with each prescription that the necessary measures have been taken, as described above.
For mentaking talidomida
Talidomida passes into the semen. Therefore, do not have unprotected sex, even if you have had a vasectomy.
- You must avoid pregnancy and any exposure during pregnancy. Always use a condom:
- During treatment.
- For at least 7 days after finishing treatment.
- Do not donate semen:
- During treatment.
- For at least 7 days after finishing treatment.
For all patients
Before taking talidomida, consult your doctor if:
- You do not understand the instructions on contraception given by your doctor or do not think you can follow them.
- You have had a heart attack, have ever had a blood clot, or if you smoke, or have high blood pressure or high cholesterol levels. During treatment with talidomida, you have a higher risk of developing blood clots in the veins and arteries (see also section 4 "Possible side effects");
- You have had or have neuropathy, i.e., nerve damage that causes tingling, abnormal coordination, or pain in the hands or feet (see also section 4 "Possible side effects").
- You have had or have a slow heart rate (may be a symptom of bradycardia).
- You have high blood pressure in the arteries that come out of the lungs (see also section 4 "Possible side effects").
- You suffer from a decrease in the number of white blood cells (neutropenia) accompanied by fever and infection.
- You suffer from a decrease in the number of platelets. You will be more prone to bleeding and bruising.
- You have or have had any liver damage (liver disorders), such as abnormal liver test results.
- You have had severe skin reactions that can start as a skin rash in one area but spread with blisters on the skin and mucous membranes (Stevens-Johnson syndrome and toxic epidermal necrolysis). You may also have a fever at the same time.
- You have had an allergic reaction while taking talidomida, such as a rash, itching, swelling, dizziness, or breathing problems.
- You have had drowsiness.
- You have had fever, chills, and intense shivering, and possibly complicated by low blood pressure and confusion (may be symptoms of severe infections).
- You have or have had any viral infection, in particular, chickenpox, hepatitis B, or HIV. If in doubt, consult your doctor. Treatment with talidomida may cause the virus to reactivate in patients who carry it, leading to the recurrence of the infection. Your doctor must check if you have ever had a hepatitis B infection;
- You have kidney or liver problems (see also section 4 "Possible side effects");
Your doctor may check if you have a high total tumor burden throughout the body, including the bone marrow. This could lead to a condition in which the tumors break down and produce abnormal levels of chemicals in the blood that can cause kidney failure (this condition is called tumor lysis syndrome) (see also section 4 "Possible side effects").
Your doctor must evaluate if you have other types of malignant hematological neoplasias (called acute myeloid leukemia and myelodysplastic syndromes) during your treatment with talidomida (see also section 4 "Possible side effects").
You must not donate blood during treatment with talidomida or for at least 7 days after finishing treatment.
If you are unsure if any of the above points apply to you, consult your doctor before starting to take talidomida.
Children and adolescents
The use of talidomida is not recommended in children and adolescents under 18 years of age.
Use of Talidomida Accord with other medicines
Tell your doctor or pharmacist if you are using or have recently used any other medication, including those obtained without a prescription and herbal preparations.
Make sure to inform your doctor if you are taking any medication that:
- Causes drowsiness, as talidomida may increase this effect. These medications include sedatives (such as anxiolytics, hypnotics, antipsychotics, antihistamines H1, opioids, and barbiturates).
- Slows down the heart rate (induces bradycardia, such as anticholinesterases and beta-blockers).
- Is used for heart problems (such as digoxin) or to thin the blood (such as warfarin).
- Is associated with neuropathy, such as other cancer treatments.
- Is used for contraception.
Taking Talidomida Accord with food, drinks, and alcohol
Do not drink alcohol if you are taking talidomida. The reason is that alcohol will make you drowsy and talidomida may make you even drowsier.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medication.
Pregnancy
Talidomida causes severe birth defects and fetal death.
- The fact that a pregnant woman takes even a small dose, such as one capsule, can cause the baby to be born with severe birth defects.
- These defects can include shorter arms or legs, deformities in hands or feet, problems with the eyes, ears, or internal organs.
If you are pregnant, do not take talidomida. Additionally, do not become pregnant if you are taking talidomida.
Women who can become pregnant must use an effective contraceptive method (see section 2, "What you need to know before taking Talidomida Accord").
You must stop treatment and inform your doctor immediately if:
- You do not have a period or think you have not had a period, in case of unusual menstrual bleeding or if you suspect you may be pregnant.
- You have unprotected heterosexual sex without using an effective contraceptive method.
If you become pregnant during treatment with talidomida, you must stop treatment and inform your doctor immediately.
Men taking talidomida and having a partner who can become pregnant must read section 2 "What you need to know before taking Talidomida Accord". If your partner becomes pregnant while you are taking talidomida, you must inform your doctor immediately.
Breastfeeding
Do not breastfeed when taking talidomida, as it is not known if talidomida passes into human breast milk.
Driving and using machines
Do not drive or use tools or machines if you experience side effects such as dizziness, fatigue, drowsiness, or blurred vision.
This medication contains Isomalt.
If your doctor has told you that you have an intolerance to certain sugars, consult with them before taking this medication.
This medication contains less than 23 mg of sodium (1 mmol) per capsule, which is essentially "sodium-free".
3. How to take Talidomida Accord
Follow your doctor's or pharmacist's instructions for taking talidomida exactly. If in doubt, consult your doctor or pharmacist again.
How much to take
The recommended dose is 200 mg (4 capsules of 50 mg) per day in adults 75 years or younger or 100 mg (2 capsules of 50 mg) per day in adults over 75 years. However, your doctor will choose the most suitable dose for you, monitor your progress, and may adjust the dose. Your doctor will indicate how you should take talidomida and for how long you will need to take it (see section 2, "What you need to know before taking Talidomida Accord").
Talidomida is taken every day in treatment cycles, each lasting 6 weeks, in combination with melfalan and prednisona, which are taken on days 1 to 4 of each 6-week cycle.
How to take this medication
- Do not break, open, or chew the capsules. If the powder from a broken talidomida capsule comes into contact with the skin, wash the skin immediately and carefully with water and soap.
- Take this medication orally.
- Swallow the capsules whole with a full glass of water.
- Do not crush or chew them.
- Take the capsules as a single dose before going to bed. This will make you feel less drowsy at other times.
To remove the capsule from the blister, press only one end of the capsule to make it come out through the foil. Do not press in the center of the capsule, as it may break.
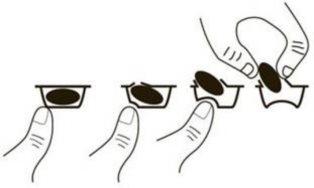
If you take more Talidomida Accord than you should
If you take a larger amount of talidomida than indicated, consult a doctor or go directly to a hospital. If possible, take the medication package and this package leaflet with you. In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, 91 562 04 20, indicating the medication and the amount ingested.
If you forget to take Talidomida Accord
If you forget to take talidomida at the usual time and
- Less than 12 hours have passed: take the capsules immediately.
- More than 12 hours have passed: do not take the capsules. Take the next capsules at the usual time the next day.
If you have any other questions about the use of this medication, ask your doctor, pharmacist, or nurse.
4. Possible Adverse Effects
Like all medicines, this medicine can cause adverse effects, although not all people suffer from them.
This medicine may cause the following adverse effects:
Stop taking thalidomide and consult a doctor immediately if you notice the following serious adverse effects – you may need urgent medical treatment:
- Severe skin reactions, such as skin rashes, which is a common adverse effect, and blisters on the skin and mucous membranes (Stevens-Johnson syndrome and toxic epidermal necrolysis, which are rare adverse effects). You may have a high temperature (fever) at the same time.
Consult your doctor immediately if you notice any of the following serious adverse effects:
- Numbness, tingling, abnormal coordination, or pain in the hands and feet.
This may be due to nerve damage (peripheral neuropathy), which is a very frequent adverse effect. It can become very severe, painful, and disabling. If you experience these symptoms, consult your doctor immediately, who may reduce the dose or interrupt treatment. This adverse effect usually occurs after taking this medicine for several months, but it can happen before. It can also happen sometime after the end of treatment. It may not disappear or may do so slowly.
- Sudden chest pain or difficulty breathing.
This may be due to blood clots in the arteries that go to the lungs (pulmonary embolism), which is a frequent adverse effect. It can appear during treatment or once treatment has ended.
- Pain or inflammation in the legs, especially in the lower part or in the calves.
This may be due to blood clots in the veins of the legs (deep vein thrombosis), which is a frequent adverse effect. It can appear during treatment or once treatment has ended.
- Chest pain that spreads to the arms, neck, jaw, back, or stomach, sweating, and shortness of breath, nausea, or vomiting.
These may be symptoms of a heart attack/myocardial infarction (which can be due to blood clots in the heart arteries).
- Temporary difficulty seeing or speaking.
These may be symptoms of a stroke (which can be due to a blood clot in a brain artery).
- Fever, chills, sore throat, cough, mouth ulcers, or any other symptom of infection.
- Bleeding or bruising in the absence of injury.
Other adverse effects include:
It is important to note that a small number of patients with multiple myeloma may develop other types of cancer, especially malignant hematological neoplasms, and it is possible that this risk increases with thalidomide treatment; therefore, your doctor should carefully evaluate the benefits and risks when prescribing thalidomide.
Very common(may affect more than 1 in 10 people)
- Constipation.
- Dizziness.
- Drowsiness, fatigue.
- Tremors.
- Decreased sensations or abnormal sensations (dysesthesia).
- Inflammation of hands and feet.
- Low blood cell counts, which could mean a higher probability of developing infections. Your doctor may monitor your blood cell counts during thalidomide treatment.
Common(may affect up to 1 in 10 people)
- Indigestion, nausea, vomiting, dry mouth.
- Rash, dry skin.
- Decrease in the number of white blood cells (neutropenia) accompanied by fever and infection.
- Decrease in the number of red blood cells, white blood cells, and platelets at the same time (pancytopenia).
- Weakness, fainting, or instability, lack of energy or strength, low blood pressure.
- Fever, general malaise.
- Seizures.
- Feeling that the head is spinning, which makes it difficult to stand and move normally.
- Blurred vision.
- Pneumonia, lung disease.
- Slow heart rate, heart failure.
- Depression, confusion, mood changes, anxiety.
- Decreased hearing or deafness.
- Kidney disease (kidney failure).
Uncommon(may affect up to 1 in 100 people)
- Inflammation and swelling of the pulmonary tubes (bronchitis).
- Inflammation of the stomach wall.
- Perforation of the large intestine (colon), which can cause an infection.
- Intestinal obstruction.
- Decrease in blood pressure when standing, which can cause fainting.
- Irregular heartbeat (heart block or atrial fibrillation), feeling of loss of consciousness or fainting.
Frequency not known(cannot be estimated from the available data):
- Underactive thyroid (hypothyroidism).
- Intestinal obstruction.
- Sexual dysfunction, for example, impotence.
- Severe blood infection (septicemia) accompanied by fever, chills, and severe tremors, and possibly complicated by low blood pressure and confusion (septic shock).
- Tumor lysis syndrome – metabolic complications that can occur during cancer treatment and sometimes even without treatment. These complications are due to the breakdown products of dying cancer cells and may include changes in blood biochemistry; high levels of potassium, phosphorus, uric acid, and low levels of calcium, which, as a consequence, produce changes in kidney function, heart rate, seizures, and sometimes death.
- Allergic reactions such as localized or generalized pruritic exanthema and angioedema (types of allergic reactions that can manifest as hives, skin rashes, eye, mouth, or face inflammation, difficulty breathing, or itching).
- Liver damage (liver disorder) including abnormal liver function test results.
- Bleeding in the stomach or intestines (gastrointestinal bleeding).
- Worsening of Parkinson's disease symptoms (such as tremors, depression, or confusion).
- Pain in the upper abdomen and/or back, which can be intense and last for several days, possibly accompanied by nausea, vomiting, fever, and rapid pulse – these symptoms may be due to pancreas inflammation (pancreatitis).
- Increased blood pressure in the blood vessels that supply blood to the lungs, which can lead to difficulty breathing, fatigue, dizziness, chest pain, faster heart rate, or swelling of the legs or ankles (pulmonary hypertension).
- Viral infections, including herpes zoster (also called "shingles", a viral disease that produces a painful rash with blisters) and recurrence of hepatitis B infection (which can cause the skin and eyes to turn yellow, dark brown urine, stomach pain on the right side, fever, nausea, or vomiting).
- A brain disease with symptoms such as changes in vision, headache, seizures, and confusion, with or without high blood pressure (posterior reversible encephalopathy syndrome or PRES).
- A disease that affects the skin caused by inflammation of small blood vessels, accompanied by joint pain and fever (leukocytoclastic vasculitis).
Reporting of adverse effects
If you experience any type of adverse effect, consult your doctor or pharmacist, even if it is a possible adverse effect that does not appear in this leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: www.notificaRAM.es. By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Storage of Thalidomide Accord
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiration date that appears on the packaging and on the blister, after CAD. The expiration date is the last day of the month indicated.
Do not use this medicine if you observe deterioration or signs of tampering with the guarantee seal.
Do not store at a temperature above 30°C
At the end of your treatment, you must return all unused capsules to the pharmacist or doctor, to prevent misuse.
6. Package contents and additional information
Composition of Thalidomide Accord:
- The active ingredient is thalidomide. Each capsule contains 50 mg of thalidomide.
- The other excipients are isomalt (E953), sodium croscarmellose, and sodium fumarate and stearate. The capsule shell contains gelatin and titanium dioxide (E171).
Appearance of the product and package contents
The hard capsules of Thalidomide Accord are white and opaque, size 4. The capsules are filled with a white powder.
PVC/PCTFE/aluminum blister pack containing 14 capsules. Package size: Diphtheria with 28 capsules (2 blister packs)
Single-dose PVC/PCTFE/aluminum blister pack containing 7 capsules. Package size: 28 capsules (4 blister packs) in boxes.
Marketing authorization holder and manufacturer
Marketing authorization holder
Accord Healthcare, S.L.U.
World Trade Center,
Moll de Barcelona, s/n,
Edifici Est, 6ª planta,
08039 Barcelona
Spain
Manufacturer
Ardena Pamplona, S.L.
Pol Mocholi, Calle Noáin 1
31110 Noáin, Navarra
Spain
O
Medichem S.A.
Narcis Monturiol, 41A
08970 Sant Joan Despí - Barcelona.
Spain
O
Bluepharma – Indústria Farmacêutica, S.A.
Eiras, Rua Adriano Lucas
Coimbra, 3020-430
Portugal
O
Bluepharma – Indústria Farmacêutica, S.A.
- Martinho do Bispo
Coimbra, 3045-016
Portugal
This medicine is authorized in the Member States of the European Economic Area under the following names:
Cyprus Thalidomide Accord 50mg σκληρ? καψ?κια
Denmark Thalidomide Accord
Slovenia Talidomid Accord 50mg trde kapsule
Spain Talidomida Accord 50 mg cápsulas duras EFG
France Thalidomide Accord 50mg gélule
Hungary Thalidomide Accord 50mg kemény kapszula
Italy Talidomide Accord
Malta Thalidomide Accord 50mg hard capsules
Poland Thalidomide Accord
Portugal Thalidomide Accord 50mg cápsulas
Romania Talidomida Accord 50 mg capsule
Date of the last revision of this leaflet: 02/2022
Detailed information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products: http://www.aemps.gob.es.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to TALIDOMIDE ACCORD 50 mg HARD CAPSULESDosage form: CAPSULE, 50 mgActive substance: thalidomideManufacturer: Bristol-Myers Squibb Pharma EeigPrescription requiredDosage form: INJECTABLE, 10 mg/ 1 mlActive substance: methotrexateManufacturer: Ebewe Pharma Ges.M.B.H. Nfg.KgPrescription requiredDosage form: INJECTABLE, 15 mgActive substance: methotrexateManufacturer: Ebewe Pharma Ges.M.B.H. Nfg.KgPrescription required
Online doctors for TALIDOMIDE ACCORD 50 mg HARD CAPSULES
Discuss questions about TALIDOMIDE ACCORD 50 mg HARD CAPSULES, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions






