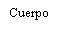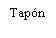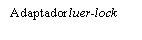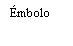SYNFLORIX SUSPENSION FOR INJECTION IN PRE-FILLED SYRINGE

How to use SYNFLORIX SUSPENSION FOR INJECTION IN PRE-FILLED SYRINGE
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Synflorix injectable suspension in pre-filled syringe
pneumococcal conjugate polysaccharide vaccine (adsorbed)
Read all of this leaflet carefully before your child is given this vaccine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This vaccine has been prescribed for your child. Do not pass it on to others.
- If your child experiences any side effects, consult your doctor or pharmacist, even if they are not listed in this leaflet. See section 4.
Contents of the pack
- What Synflorix is and what it is used for
- What you need to know before your child is given Synflorix
- How Synflorix is given
- Possible side effects
- Storing Synflorix
- Contents of the pack and other information
1. What Synflorix is and what it is used for
Synflorix is a pneumococcal conjugate vaccine. Your doctor or nurse will inject this vaccine into your child.
It is used to help protect your child from 6 weeks up to 5 years of age against:
a bacterium called Streptococcus pneumoniae. This bacterium can cause serious diseases including meningitis, sepsis, and bacteremia (bacteria in the bloodstream), as well as ear infections or pneumonia.
How Synflorix works
Synflorix helps the body to produce its own antibodies. Antibodies are part of the immune system that will protect your child from these diseases.
2. What you need to know before your child is given Synflorix
Synflorix must not be given if
- your child is allergic to the active substance or to any of the other ingredients of this vaccine (listed in section 6).
Signs of an allergic reaction may include skin rash with itching, difficulty breathing, and swelling of the face or tongue.
- your child has a severe infection with high fever (over 38 °C). If this happens to your child, vaccination should be delayed until they feel better. A minor infection, such as a cold, should not be a problem for vaccination. However, consult your doctor before.
Synflorix must not be given if your child is in any of the above situations. If you are not sure, consult your doctor or pharmacist before your child is given Synflorix.
Warnings and precautions
Consult your doctor or pharmacist before starting to use this vaccine if:
- your child has any bleeding problems or bruises easily.
In children from 2 years of age, before or after any injection, fainting may occur, so you should inform your doctor or nurse if your child has fainted after previous injections.
Like all vaccines, Synflorix may not completely protect all vaccinated children.
Synflorix will only protect against infections caused by the bacteria for which the vaccine has been developed.
Children with a weakened immune system (e.g., due to HIV infection or immunosuppressive treatment) may not fully benefit from Synflorix.
If you are not sure, talk to your doctor or pharmacist before using Synflorix.
Children over 5 years
The safety and efficacy of the vaccine have not been established in children over 5 years, so vaccination of these children is not recommended.
Other medicines and Synflorix
Tell your doctor or pharmacist if your child is taking, has recently taken, or might take any other medicines, or if they have recently been given any other vaccine. Synflorix may not work as well if your child is taking medicines that affect the immune system to fight an infection.
Synflorix can be given at the same time as other childhood vaccines, such as vaccines against diphtheria, tetanus, pertussis (whooping cough), Haemophilus influenzaetype b, oral or inactivated polio, hepatitis B, measles, mumps, and rubella (MMR) vaccine, varicella, oral rotavirus vaccines, and conjugate vaccines against meningococcal serogroup C and serogroups A, C, W-135, and Y. A different injection site will be used for each vaccine.
Your doctor may recommend giving your child a medicine to reduce fever (such as paracetamol) before or immediately after receiving Synflorix, especially in children who have been vaccinated at the same time with Synflorix and other vaccines that contain whole-cell pertussis. It is also recommended to give a medicine to reduce fever in children with seizure disorders or a history of febrile seizures.
However, if your child has received paracetamol before or immediately after receiving Synflorix, the levels of antibodies obtained may be slightly reduced. It is not known if the reduction in antibody levels has an impact on protection against pneumococcal diseases.
Synflorix contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per dose, which is essentially "sodium-free".
3. How Synflorix is given
How to use the vaccine
Synflorix is always injected into a muscle. Usually in the thigh or upper arm.
How much to use
Generally, your child (from 6 weeks to 6 months of age) will receive a series of 4 injections according to official recommendations, but the healthcare professional may use a different vaccination schedule. It is important to follow the doctor's or nurse's instructions to complete the series of injections.
- Each injection will be given at least one month apart, except for the last injection (booster), which will be given at least six months after the third injection.
- The first injection can be given from 6 weeks of age. The last injection (booster) can be given from 9 months of age.
- You will be informed when your child needs to come back for the next injections.
Premature infants (born after 27 weeks and before 37 weeks of gestation):
Your child (from 2 months to 6 months of age) will receive 3 injections with an interval of at least one month between each dose. Your child will receive an additional injection (booster) at least six months after the last injection.
Infants from 7 to 11 months of age will receive 2 injections. Each injection will be given at least one month apart. A third injection (booster) will be given during the second year of life with at least two months between them.
Children from 12 months to 5 years will receive 2 injections. Each injection will be given at least two months apart.
Special populations:
Children from 6 weeks to 5 years of age with a higher risk of pneumococcal infection (such as those with HIV infection, sickle cell anemia, or damaged or non-functioning spleen) may receive Synflorix. Please consult your doctor for information about the number and timing of injections for your child.
If your child misses an injection
If your child misses an injection, it is important that you schedule another appointment. This way, you and your doctor can discuss the steps to be taken to protect your child.
If you have any other questions about the use of this vaccine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this vaccine can cause side effects, although not everybody gets them. The following side effects may occur with this vaccine:
Very rare serious allergic reactions (up to 1 in 10,000 doses of the vaccine) may occur. These reactions can be recognized by:
- itchy lumps (hives)
- swelling, sometimes of the face or mouth (angioedema), which causes difficulty breathing
- fainting.
These reactions usually occur before leaving the doctor's office. In any case, if your child has any of these symptoms, contact a doctor urgently.
Very common(may occur with more than 1 in 10 doses of the vaccine): pain, redness, and swelling at the injection site, high temperature, 38 °C or higher (fever), sleepiness, irritability, loss of appetite.
Common(may occur with up to 1 in 10 doses of the vaccine): hardening of the injection site.
Uncommon(may occur with up to 1 in 100 doses of the vaccine): itching, appearance of blood clots, bleeding, or small lumps at the injection site, nausea, diarrhea, or discomfort (vomiting), unusual crying, temporary stops in breathing (apnea) if your child was born prematurely (at 28 weeks of gestation or earlier), headache, skin rash, diffuse swelling of the injected limb, sometimes affecting the adjacent joint, hives.
Rare(may occur with up to 1 in 1,000 doses of the vaccine): seizures without high temperature or due to high temperature (fever), allergic reactions such as skin allergies, collapse (sudden muscle weakness), periods of unconsciousness or loss of consciousness, and paleness or change in skin color to a bluish tone.
Very rare(may occur with up to 1 in 10,000 doses of the vaccine): Kawasaki disease (some of the serious signs of the disease are: fever, skin rash, swelling of the lymph nodes, inflammation, and rash of the mucous membranes of the mouth and throat).
Booster doses of Synflorix may increase the risk of side effects.
For children over 12 months, the risk of pain at the injection site may increase with age.
In very premature children (born at 28 weeks of gestation or earlier) during the 2-3 days following vaccination, the periods between breaths may be longer than usual.
Reporting of side effects
If your child experiences any side effects, consult your doctor or pharmacist, even if they are not listed in this leaflet. You can also report them directly through the national reporting system included in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storing Synflorix
Keep this medicine out of the sight and reach of children.
- Do not use this medicine after the expiry date which is stated on the carton. The expiry date is the last day of the month shown.
- Store in a refrigerator (between 2 °C and 8 °C).
- Store in the original package to protect from light.
- Do not freeze.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Package Contents and Additional Information
Composition of Synflorix
- The active ingredients are:
A 0.5 ml dose contains:
Pneumococcal serotype 11 polysaccharide, 1 microgram
Pneumococcal serotype 4 polysaccharide, 3 micrograms
Pneumococcal serotype 5 polysaccharide, 1 microgram
Pneumococcal serotype 6B polysaccharide, 1 microgram
Pneumococcal serotype 7F polysaccharide, 1 microgram
Pneumococcal serotype 9V polysaccharide, 1 microgram
Pneumococcal serotype 14 polysaccharide, 1 microgram
Pneumococcal serotype 18C polysaccharide, 3 micrograms
Pneumococcal serotype 19F polysaccharide, 3 micrograms
Pneumococcal serotype 23F polysaccharide, 1 microgram
1 adsorbed on aluminum phosphate, 0.5 milligrams of Al3+ in total
2 conjugated with protein D (derived from non-typable Haemophilus influenzae) as a carrier protein, 9-16 micrograms
3 conjugated with tetanus toxoid as a carrier protein, 5-10 micrograms
4 conjugated with diphtheria toxoid as a carrier protein, 3-6 micrograms
- The other components are: sodium chloride (for more information, see section 2) and water for injectable preparations
Appearance and Package Contents of the Product
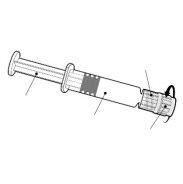
- Injectable suspension in a pre-filled syringe
- Synflorix is a turbid white suspension.
- Synflorix is available in a pre-filled syringe of 1 dose, with or without separate needles; package sizes of 1, 10, and 50.
- Only some package sizes may be marketed.
Marketing Authorization Holder and Manufacturer
GlaxoSmithKline Biologicals s.a.
Rue de l’Institut 89
B-1330 Rixensart
Belgium
For more information about this medicinal product, please contact the local representative of the marketing authorization holder:
Belgium/Belgi/Belgien GlaxoSmithKline Pharmaceuticals s.a./n.v. Tel: + 32 10 85 52 00 | Lithuania GlaxoSmithKline Biologicals SA Tel: +370 80000334 |
Bulgaria GlaxoSmithKline Biologicals SA Tel: +359 80018205 | Luxembourg/Luxemburg GlaxoSmithKline Pharmaceuticals s.a./n.v. Tel: + 32 10 85 52 00 |
Czech Republic GlaxoSmithKline s.r.o. Tel: + 420 2 22 00 11 11 | Hungary GlaxoSmithKline Biologicals SA Tel: +36 80088309 |
Denmark GlaxoSmithKline Pharma A/S Tel: + 45 36 35 91 00 | Malta GlaxoSmithKline Biologicals SA Tel: +356 80065004 |
Germany GlaxoSmithKline GmbH & Co. KG Tel: + 49 (0)89 360448701 | Netherlands GlaxoSmithKline BV Tel: + 31 (0)33 2081100 |
Estonia GlaxoSmithKline Biologicals SA Tel: +372 8002640 | Norway GlaxoSmithKline AS Tel: + 47 22 70 20 00 |
Greece GlaxoSmithKline Μονοπρ?σωπη A.E.B.E Tel: + 30 210 68 82 100 | Austria GlaxoSmithKline Pharma GmbH. Tel: + 43 (0)1 97075 0 |
Spain GlaxoSmithKline, S.A. Tel: + 34 900 202 700 | Poland GSK Services Sp. z o.o. Tel: + 48 (22) 576 9000 |
France Laboratoire GlaxoSmithKline Tel: + 33 (0) 1 39 17 84 44 | Portugal GlaxoSmithKline - Produtos Farmacêuticos, Lda. Tel: + 351 21 412 95 00 |
Croatia GlaxoSmithKline Biologicals SA Tel: +385 800787089 | Romania GlaxoSmithKline Biologicals SA Tel: +40 800672524 |
Ireland GlaxoSmithKline (Ireland) Ltd Tel: + 353 (0)1 495 5000 | Slovenia GlaxoSmithKline Biologicals SA Tel: +386 80688869 |
Iceland Vistor hf. Tel: +354 535 7000 | Slovak Republic GlaxoSmithKline Biologicals SA Tel: +421 800500589 |
Italy GlaxoSmithKline S.p.A. Tel: + 39 (0)45 7741 111 | Finland GlaxoSmithKline Oy Tel: + 358 10 30 30 30 |
Cyprus GlaxoSmithKline Biologicals SA Tel: +357 80070017 | Sweden GlaxoSmithKline AB Tel: + 46 (0)8 638 93 00 |
Latvia GlaxoSmithKline Biologicals SA Tel: +371 80205045 | United Kingdom (Northern Ireland) GlaxoSmithKline Biologicals SA Tel: +44 (0)800 221 441 |
Date of Last Revision of this Leaflet:
Other Sources of Information
Detailed information on this medicinal product is available on the European Medicines Agency website http://www.ema.europa.eu.
This information is intended only for healthcare professionals:
During storage of the pre-filled syringe, a fine white deposit with a clear transparent supernatant may be observed. This does not constitute a sign of deterioration.
Before administration, the contents of the pre-filled syringe should be visually inspected both before and after shaking for any foreign particles and/or variation in physical appearance. If any of these circumstances are observed, discard the vaccine.
The vaccine should be allowed to reach room temperature before use.
The vaccine should be well shaken before use.
The vaccine is intended for intramuscular administration only. Do not administer intravascularly.
If Synflorix is administered with other vaccines, different injection sites should be used.
Synflorix should not be mixed with other vaccines.
Instructions for the pre-filled syringe
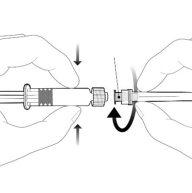
| Hold the syringe by the body, not by the plunger. Unscrew the syringe cap by turning it counterclockwise. |
| To insert the needle, connect the base to the Luer-Lock adapter and turn it one-quarter turn clockwise until it clicks. Do not pull the plunger out of the syringe body. If this happens, do not administer the vaccine. |
Disposal of Waste
Disposal of the unused product and all materials that have come into contact with it should be done in accordance with local regulations.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to SYNFLORIX SUSPENSION FOR INJECTION IN PRE-FILLED SYRINGEDosage form: INJECTABLEManufacturer: Glaxosmithkline BiologicalsPrescription requiredDosage form: INJECTABLE, 4 micrograms/doseActive substance: pneumococcus, purified polysaccharides antigen conjugatedManufacturer: Merck Sharp & Dohme B.V.Prescription requiredDosage form: INJECTABLE, 25 micrograms of each serotype/0.5mlActive substance: pneumococcus, purified polysaccharides antigenManufacturer: Merck Sharp & Dohme De Espana S.A.Prescription required
Online doctors for SYNFLORIX SUSPENSION FOR INJECTION IN PRE-FILLED SYRINGE
Discuss questions about SYNFLORIX SUSPENSION FOR INJECTION IN PRE-FILLED SYRINGE, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions