SYMTUZA 800 mg/150 mg/200 mg/10 mg FILM-COATED TABLETS

How to use SYMTUZA 800 mg/150 mg/200 mg/10 mg FILM-COATED TABLETS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Symtuza 800 mg/150 mg/200 mg/10 mg film-coated tablets
darunavir/cobicistat/emtricitabine/tenofovir alafenamide
This medicine is subject to additional monitoring, which will help to quickly identify new safety information. You can help by reporting any side effects you may get. The last section of this leaflet includes information on how to report side effects.
Read all of this leaflet carefully before you start taking this medicine, because
it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only, and you should not pass it on to others.
even if they have the same symptoms as you, as it may harm them.
- If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if it is not listed in this leaflet. See section 4.
Contents of the pack
- What is Symtuza and what is it used for
- What you need to know before you take Symtuza
- How to take Symtuza
- Possible side effects
- Storage of Symtuza
- Contents of the pack and further information
1. What is Symtuza and what is it used for
Symtuza is an antiretroviral medicine used to treat human immunodeficiency virus type 1 (HIV-1) infection. It is used in adults and adolescents from 12 years of age and weighing at least 40 kilograms. Symtuza contains four active substances:
- darunavir, an HIV medicine known as a protease inhibitor
- cobicistat, a booster (enhancer) of darunavir
- emtricitabine, an HIV medicine known as a nucleoside reverse transcriptase inhibitor
- tenofovir alafenamide, an HIV medicine known as a nucleotide reverse transcriptase inhibitor
Symtuza reduces the HIV-1 virus in your body, and this will improve your immune system (the body's natural defenses) and reduce the risk of you developing illnesses related to HIV infection, although Symtuza is not a cure for HIV infection.
2. What you need to know before you take Symtuza
Do not take Symtuza
- if you are allergic(hypersensitive) to darunavir, cobicistat, emtricitabine, tenofovir alafenamide, or any of the other ingredients of this medicine (listed in section 6).
- if you have severe liver problems. Ask your doctor if you are unsure about the severity of your liver disease. You may need to have some additional tests.
Do not combine Symtuza with any of the following medicines
If you are taking any of these, consult your doctor about the possibility of switching to another medicine.
Medicine | Indication of the medicine |
Alfuzosin | to treat enlarged prostate |
Amiodarone, dronedarone, ivabradine, quinidine, or ranolazine | to treat certain heart rhythm disorders (e.g., irregular heartbeat) |
Carbamazepine, phenobarbital, and phenytoin | to prevent seizures |
Colchicine (if you have kidney or liver problems) | to treat gout |
Lopinavir/ritonavir combination product | HIV medicine |
Rifampicin | to treat certain infections such as tuberculosis |
Pimozide, lurasidone, quetiapine, or sertindol | to treat psychiatric disorders |
Ergot alkaloids such as ergotamine, dihydroergotamine, ergometrine, and methylergonovine | to treat migraines |
St. John's Wort (Hypericum perforatum) | herbal medicine used for depression |
Lovastatin, simvastatin, and lomitapide | to lower cholesterol levels |
Triazolam or midazolam (taken by mouth) | to help you sleep and/or to relieve anxiety |
Sildenafil | to treat a heart and lung condition called pulmonary arterial hypertension. Sildenafil has other uses. See the section "Other medicines and Symtuza". |
Avanafil | to treat erectile dysfunction |
Dabigatran, ticagrelor | to help prevent blood clots in patients who have had a heart attack |
Naloxegol | to treat opioid-induced constipation |
Dapoxetine | to treat premature ejaculation |
Domperidone | to treat nausea and vomiting |
Warnings and precautions
Talk to your doctor, pharmacist, or nurse before you start taking Symtuza.
While you are taking this medicine, you can still pass on HIV to others, although the risk is reduced with effective antiretroviral treatment. Ask your doctor about the precautions you need to take to avoid infecting others.
People taking Symtuza may still develop infections and other illnesses associated with HIV. You should keep in regular contact with your doctor.
People taking Symtuza may develop a skin rash. In rare cases, a rash can be severe or even life-threatening. If you develop a rash, please contact your doctor.
Talk to your doctor before taking Symtuza. Inform your doctor immediately if you are in any of the following situations.
- If you have had liver problems, including hepatitis B or C infection. Your doctor will assess the severity of your liver disease before deciding whether you can take Symtuza.
- If you have hepatitis B, your liver problems may get worse when you stop taking Symtuza. It is important that you do not stop taking Symtuza without talking to your doctor first.
- If you have had kidney problems. Your doctor will consider whether Symtuza is suitable for you.
- If you have diabetes. Symtuza may increase your blood sugar levels.
- If you notice any symptoms of infection(e.g., swollen lymph nodes and fever). Some patients with advanced HIV infection and a weakened immune system (opportunistic infections) may develop signs and symptoms of inflammation from previous infections soon after starting HIV treatment. It is believed that these symptoms are due to the improvement in the body's immune response, enabling it to fight off infections that may not have caused symptoms before.
- If you notice symptoms such as muscle weakness, weakness starting in the hands and feet and moving towards the trunk, palpitations, tremors, or hyperactivity, tell your doctor immediately. In addition to opportunistic infections, you may also develop autoimmune disorders(when the immune system attacks the body's healthy tissues) due to the improvement in the body's immune response after starting HIV treatment. Autoimmune disorders may occur many months after starting treatment.
- If you have hemophilia. Symtuza may increase the risk of bleeding.
- If you are allergic to sulfonamides(used, for example, to treat certain infections).
- If you notice any muscle or bone problems. Some patients taking HIV medicines may develop a bone disease called osteonecrosis (damage to bone tissue caused by loss of blood supply to the bones). This is more likely with long-term HIV treatment, when there is more damage to the immune system, you are overweight, or you use alcohol or medicines called corticosteroids. The signs of osteonecrosis are joint stiffness, pain, and difficulty moving. If you notice any of these symptoms, talk to your doctor.
Elderly patients
Symtuza has only been used in a small number of patients aged 65 years or older. If you belong to this age group, talk to your doctor to see if you can use Symtuza.
Children and adolescents:
Symtuza must not be used in children under 12 years of age or weighing less than 40 kg, as it has not been studied in children under 12 years of age.
Other medicines and Symtuza
Tell your doctor or pharmacist if you are taking or have recently taken any other medicines.
Some medicines must not be takenwith Symtuza. These medicines are listed above in the section "Do not combine Symtuza with any of the following medicines:"
Symtuza must not be used with other antiviral medicines that contain a booster or with other antivirals that require boosting. In some cases, it may be necessary to adjust the dose of the other medicines. Therefore, always inform your doctor if you are taking any other HIV medicine and follow your doctor's instructions carefully about which medicines can be combined.
Additionally, do not take Symtuza with medicines that contain tenofovir disoproxil (such as fumarate, phosphate, or succinate), lamivudine, or adefovir dipivoxil, or with medicines that require boosting with ritonavir or cobicistat.
The effects of Symtuza may be reduced if you take any of the following products. Inform your doctor if you are taking:
- Bosentan(to treat high blood pressure in the pulmonary circulation)
- Dexamethasone(injection) (corticosteroid)
- Rifapentine, rifabutin(to treat bacterial infections)
- Oxcarbazepine(to prevent seizures).
Symtuza may also affect the effects of other medicines. Inform your doctor if you are taking:
- Amlodipine, diltiazem, disopyramide, felodipine, flecainide, mexiletine, nicardipine, nifedipine, propafenone, lidocaine, verapamil(for heart diseases), as the therapeutic effect or side effects of these medicines may be increased.
- Bosentan(to treat high blood pressure in the pulmonary circulation)
- Apixaban, edoxaban, rivaroxaban, clopidogrel(to reduce blood clotting), as the therapeutic effect or side effects may be modified.
- Clonazepam(to prevent seizures).
- Hormonal contraceptives based on estrogensand hormone replacement therapy. Symtuza may reduce their effectiveness. When used as contraceptives, alternative non-hormonal methods of contraception are recommended.
- Ethinylestradiol/drospirenone. Symtuza may increase the risk of elevated potassium levels due to the effect of drospirenone.
- Budesonide, fluticasone(to control asthma). Should only be used after medical evaluation and under close medical supervision, in case of adverse effects of corticosteroids.
- Buprenorphine/naloxone, methadone(medicines for opioid dependence treatment)
- Salmeterol(medicine for asthma treatment)
- Artemether/lumefantrine(a combination of medicines for malaria treatment)
- Dasatinib, irinotecan, nilotinib, vinblastine, vincristine(medicines for cancer treatment)
- Prednisone(corticosteroid)
- Sildenafil, tadalafil, vardenafil(for erectile dysfunction or pulmonary arterial hypertension)
- Glecaprevir/pibrentasvir(to treat hepatitis C virus infection).
- Fentanyl, oxycodone, tramadol(to treat pain).
- Fesoterodine, solifenacin(to treat urological disorders).
It may be necessary to adjust the dose of other medicines since their therapeutic effects or side effects, or those of Symtuza, may be affected when taken together. Inform your doctor if you are taking:
- Alfentanil(a potent, short-acting injectable analgesic used in surgical procedures)
- Carvedilol, metoprolol, timolol(for heart diseases)
- Warfarin(to reduce blood clotting), as the therapeutic effect or side effects may be modified; your doctor may need to perform blood tests.
- Digoxin(to treat certain heart disorders)
- Clarithromycin(antibiotic)
- Clotrimazole, fluconazole, isavuconazole, itraconazole, posaconazole, (to treat fungal infections). Voriconazole should only be taken after medical evaluation.
- Atorvastatin, fluvastatin, pitavastatin, pravastatin, rosuvastatin(to lower cholesterol levels). There may be an increased risk of muscle damage. Your doctor will assess which cholesterol-lowering regimen is best for your individual situation.
- Rifabutin(to combat bacterial infections)
- Tadalafil, sildenafil, vardenafil(for erectile dysfunction or pulmonary arterial hypertension)
- Amitriptyline, desipramine, imipramine, nortriptyline, paroxetine, sertraline, trazodone(to treat depression and anxiety)
- Perphenazine, risperidone, thioridazine(psychiatric medicines)
- Ciclosporin, everolimus, tacrolimus, sirolimus(to suppress the immune system), as the therapeutic effect or side effects of these medicines may be increased. Your doctor may want to perform additional tests.
- Colchicine(anti-gout). If you have kidney or liver problems, see the section "Do not combine Symtuza with any of the following medicines".
- Buspirone, chlorazepate, diazepam, estazolam, flurazepam, zolpidem, midazolamwhen used as an injection (medicines used to treat sleep problems or anxiety)
- Metformin(to treat type 2 diabetes)
This list of medicines is notexhaustive. Inform your healthcare professional about allthe medicines you are taking.
Pregnancy and breastfeeding
Tell your doctor immediately if you are pregnant, plan to become pregnant, or are breastfeeding. Pregnant or breastfeeding women must not take Symtuza.
Women with HIV should not breastfeed their babies because of the risk of passing on the virus to the baby through breast milk, and because the medicine may affect the baby.
Driving and using machines
Symtuza may cause dizziness. Do not operate machinery or drive if you experience dizziness after taking Symtuza.
Symtuza contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per tablet; this is essentially "sodium-free".
3. How to take Symtuza
Follow the administration instructions of this medication exactly as indicated by your doctor or pharmacist. In case of doubt, consult your doctor or pharmacist again.
The recommended dose in adults and adolescents 12 years of age and older, who weigh at least 40 kg, is one tablet once daily with food.
You should take Symtuza daily and always with food. You should take a meal or snack within 30 minutes prior to taking Symtuza. The type of food is not important.
- The tablet should not be crushed; instead, it should be swallowed whole. The tablet can be taken with a drink, which can be water, milk, or a nutritional drink. Take Symtuza around the same time every day.
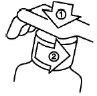
How to remove the child-resistant cap
| The plastic bottle has a child-resistant cap that is opened as follows:
|
If you take more Symtuza than you should
Contact your doctor or the nearest emergency service immediately and ask for help. Bring the bottle with you so that you can show what you have taken.
If you forget to take Symtuza
It is important that you do not miss a dose of Symtuza.
If you skip a dose:
- If you realize within 12 hoursafter the time you normally take Symtuza, take the tablet immediately with some food. Then take the next dose at the usual time.
- If you realize 12 hours or moreafter the time you normally take Symtuza, do not take the missed dose and take the next doses at the usual time, with some food. Do not take a double dose to make up for the missed doses.
If you vomit within one hour after taking the medication, you should take another dose of Symtuza with food as soon as possible. If you vomit more than 1 hour after taking the medication, you will not need to take another dose of Symtuza until the next scheduled time.
Contact your doctor if you have doubts about what to do if you forget a dose or vomit.
Do not stop taking Symtuza without talking to your doctor
HIV medications can make you feel better. Although you feel better, do not stop taking Symtuza. Consult your doctor first.
When you have little Symtuza left,ask your doctor or pharmacist for more. This is very important because the amount of virus can start to increase if you stop taking the medication, even for a short period. The disease would then be more difficult to treat.
If you have both HIV and hepatitis B infection,it is very important that you do not stop taking Symtuza without talking to your doctor first. You may need to have blood tests for several months after stopping treatment with Symtuza. In some patients with advanced liver disease or cirrhosis, worsening of hepatitis may occur after stopping treatment, which can be potentially fatal.
Inform your doctor as soon as possibleif you experience any new or unusual symptoms after stopping treatment, especially symptoms related to hepatitis B infection.
If you have any other questions about the use of this medication, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medications, this medication can cause side effects, although not everyone will experience them.
Tell your doctor if you experience any of the following side effects.
Liver problems have been reported, which can sometimes be serious. Your doctor will perform blood tests before starting treatment with Symtuza. If you have chronic hepatitis B or C infection, your doctor will repeat blood tests more frequently, as you may have a higher risk of liver problems. Talk to your doctor about the signs and symptoms of liver problems. These can include yellowing of the skin or the whites of the eyes, dark-colored urine (like tea), light-colored stools, nausea, vomiting, loss of appetite, or pain, discomfort, or tenderness in the right side below the ribs.
A skin rash may appear in more than 1 in 10 patients treated with Symtuza. Although most rashes are mild and disappear over time even if treatment continues, they can sometimes be serious or potentially life-threatening. It is essential that you talk to your doctor if you experience a rash. Your doctor will advise you on how to manage your symptoms and whether you should stop taking Symtuza.
Other serious side effects, observed in up to 1 in 10 patients, were diabetes, increased blood fat levels, and symptoms of infection. Pancreatitis (inflammation of the pancreas) has been reported in up to 1 in 100 patients.
Very common side effects (may affect more than 1 in 10 people)
- headache
- diarrhea
- skin rash
Common side effects (may affect up to 1 in 10 people)
- low red blood cell count (anemia)
- allergic reactions, such as hives, itching
- decreased appetite (anorexia)
- abnormal dreams
- vomiting, stomach pain or swelling, indigestion, flatulence (gas)
- abnormal blood test results, such as some kidney tests. Your doctor will explain these to you.
- dizziness
- joint pain
- muscle pain, cramps, or muscle weakness
- weakness
- fatigue
- feeling unwell (nausea)
Uncommon side effects (may affect up to 1 in 100 people)
- severe skin inflammation and other tissue inflammation (more common in lips or eyes)
- symptoms of infection or autoimmune disorders (immune reconstitution inflammatory syndrome)
- breast enlargement
- bone damage (osteonecrosis) caused by loss of blood supply to the bones
- abnormal blood test results, such as some pancreas tests. Your doctor will explain these to you.
Rare side effects (may affect up to 1 in 1,000 people)
- a reaction called DRESS (severe rash, which can be accompanied by fever, fatigue, swelling of the face or lymph nodes, increased eosinophils, liver, kidney, or lung effects).
Some side effects are typical of HIV medications similar to Symtuza. These include:
- high blood sugar and worsening of diabetes
- muscle pain, tenderness, or weakness. In rare cases, these muscle disorders have been serious.
- immune reconstitution inflammatory syndrome. In some patients with advanced HIV infection (AIDS) and a history of opportunistic infections, signs and symptoms of inflammation due to previous infections may appear soon after starting HIV treatment, including Symtuza. In addition to opportunistic infections, you may experience an autoimmune disorder after starting HIV medications. Autoimmune disorders can appear many months after starting treatment.
If you experience any of these symptoms, inform your doctor.
During HIV treatment, weight gain and increased lipid and glucose levels in the blood may occur. This is partly related to recovery of health and lifestyle, and in the case of blood lipids, sometimes to the HIV medications themselves.
Your doctor will perform tests to detect these changes.
Reporting side effects
If you experience any side effects, consult your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. You can also report them directly through the national reporting system included in Appendix V. By reporting side effects, you can help provide more information on the safety of this medication.
5. Storage of Symtuza
Keep this medication out of the sight and reach of children.
Do not use this medication after the expiration date stated on the carton and bottle after EXP. The expiration date is the last day of the month indicated.
Do not use this medication after 6 weeks after the first opening of the bottle.
Store in the original package to protect from moisture. Keep the bottle tightly closed.The tablets can be stored outside the original package for up to 7 days and should be discarded after that time if not taken. Tablets stored outside the original package should not be returned to the package.
Medications should not be disposed of through wastewater or household waste. Ask your pharmacist how to dispose of the packaging and any unused medication. This will help protect the environment.
6. Package contents and additional information
Symtuza composition
The active substances are darunavir, cobicistat, emtricitabine, and tenofovir alafenamide. Each film-coated tablet contains 800 mg of darunavir (as ethanolate), 150 mg of cobicistat, 200 mg of emtricitabine, and 10 mg of tenofovir alafenamide (as fumarate).
Other ingredients are:
Tablet core:
The tablet core contains sodium croscarmellose, magnesium stearate, microcrystalline cellulose, and colloidal silicon dioxide.
Tablet coating:
The film coating contains polyethylene glycol (macrogol), partially hydrolyzed polyvinyl alcohol, talc, titanium dioxide, and yellow iron oxide.
Appearance of the product and package contents
Film-coated tablet, capsule-shaped, yellow to yellow-brown, with the inscription «8121» on one side and «JG» on the other.
Symtuza is available in bottles of 30 tablets (with silica gel desiccant that should remain in the bottle to help protect the tablets). The silica gel desiccant is included in a separate bag or container and should not be ingested.
Symtuza tablets are available in packages containing one bottle or three bottles per carton.
Not all pack sizes may be marketed.
Marketing authorization holderJanssen-Cilag International NV, Turnhoutseweg 30, 2340 Beerse, Belgium
Manufacturer
Janssen-Cilag SpA, Via C. Janssen, Borgo San Michele, 04100 Latina, Italy
You can request more information about this medication by contacting the local representative of the marketing authorization holder:
België/Belgique/Belgien Janssen-Cilag NV Tel/Tél: +32 14 64 94 11 | Lietuva UAB "JOHNSON & JOHNSON" Tel: +370 5 278 68 88 |
???????? „??????? & ??????? ????????” ???? ???.: +359 2 489 94 00 | Luxembourg/Luxemburg Janssen-Cilag NV Tél/Tel: +32 14 64 94 11 |
Ceská republika Janssen-Cilag s.r.o. Tel: +420 227 012 227 | Magyarország Janssen-Cilag Kft. Tel.: +36 1 884 2858 |
Danmark Janssen-Cilag A/S Tlf: +45 4594 8282 | Malta AM MANGION LTD Tel: +356 2397 6000 |
Deutschland Janssen-Cilag GmbH Tel: +49 2137 955 955 | Nederland Janssen-Cilag B.V. Tel: +31 76 711 1111 |
Eesti UAB "JOHNSON & JOHNSON" Eesti filiaal Tel: +372 617 7410 | Norge Janssen-Cilag AS Tlf: +47 24 12 65 00 |
Ελλ?δα Janssen-Cilag Φαρμακευτικ? Α.Ε.Β.Ε. Tηλ: +30 210 80 90 000 | Österreich Janssen-Cilag Pharma GmbH Tel: +43 1 610 300 |
España Janssen-Cilag, S.A. Tel: +34 91 722 81 00 | Polska Janssen-Cilag Polska Sp. z o.o. Tel.: +48 22 237 60 00 |
France Janssen-Cilag Tél: 0 800 25 50 75 / +33 1 55 00 40 03 | Portugal Janssen-Cilag Farmacêutica, Lda. Tel: +351 214 368 600 |
Hrvatska Johnson & Johnson S.E. d.o.o. Tel: +385 1 6610 700 | România Johnson & Johnson România SRL Tel: +40 21 207 1800 |
Ireland Janssen Sciences Ireland UC Tel: +353 1 800 709 122 | Slovenija Johnson & Johnson d.o.o. Tel: +386 1 401 18 00 |
Ísland Janssen-Cilag AB c/o Vistor hf. Sími: +354 535 7000 | Slovenská republika Johnson & Johnson, s.r.o. Tel: +421 232 408 400 |
Italia Janssen-Cilag SpA Tel: 800.688.777 / +39 02 2510 1 | Suomi/Finland Janssen-Cilag Oy Puh/Tel: +358 207 531 300 |
Κ?προς Βαρν?βας Χατζηπαναγ?ς Λτδ Τηλ: +357 22 207 700 | Sverige Janssen-Cilag AB Tfn: +46 8 626 50 00 |
Latvija UAB "JOHNSON & JOHNSON" filiale Latvija Tel: +371 678 93561 | United Kingdom Janssen-Cilag Ltd. Tel: +44 1 494 567 444 |
Date of last revision of this leaflet: {MM/AAAA}.
Detailed information on this medication is available on the European Medicines Agency website: http://www.ema.europa.eu.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to SYMTUZA 800 mg/150 mg/200 mg/10 mg FILM-COATED TABLETSDosage form: TABLET, 600 mg / 300 mgActive substance: lamivudine and abacavirManufacturer: Aurovitas Spain, S.A.U.Prescription requiredDosage form: TABLET, 600 mg/300 mgActive substance: lamivudine and abacavirManufacturer: Reddy Pharma Iberia S.A.Prescription requiredDosage form: TABLET, 600 mg/300 mgActive substance: lamivudine and abacavirManufacturer: Glenmark Arzneimittel GmbhPrescription required
Online doctors for SYMTUZA 800 mg/150 mg/200 mg/10 mg FILM-COATED TABLETS
Discuss questions about SYMTUZA 800 mg/150 mg/200 mg/10 mg FILM-COATED TABLETS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions