
SYCREST 5 mg SUBLINGUAL TABLETS

How to use SYCREST 5 mg SUBLINGUAL TABLETS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Sycrest 5mg sublingual tablets
Sycrest 10mg sublingual tablets
asenapine
Read the entire package leaflet carefully before starting to take this medication, as it contains important information for you.
- Keep this package leaflet, as you may need to read it again.
- If you have any questions, consult your doctor, pharmacist, or nurse.
- This medication has been prescribed to you only, and you should not give it to others, even if they have the same symptoms as you, as it may harm them.
- If you experience side effects, consult your doctor, pharmacist, or nurse, even if they are not listed in this package leaflet. See section 4.
Contents of the Package Leaflet
- What is Sycrest and what is it used for
- What you need to know before taking Sycrest
- How to take Sycrest
- Possible side effects
- Storage of Sycrest
- Contents of the pack and further information
1. What is Sycrest and what is it used for
Sycrest contains the active ingredient asenapine. This medication belongs to a group of medications called antipsychotics. Sycrest is used to treat moderate to severe manic episodes associated with bipolar I disorder in adults. Antipsychotic medications affect the chemicals that allow communication between nerve cells (neurotransmitters). Diseases that affect the brain, such as bipolar I disorder, may be due to an imbalance of certain brain chemicals, such as dopamine and serotonin, and these imbalances can cause some of the symptoms you are experiencing. It is not known exactly how this medication works, but it is believed to adjust the balance of these chemicals.
Manic episodes associated with bipolar I disorder are a disease with symptoms such as feeling well, having excessive energy, needing to sleep much less than usual, talking very quickly with many ideas, and sometimes great irritability.
2. What you need to know before taking Sycrest
Do not take Sycrest
If you are allergic to asenapine or any of the other components of this medication (listed in section 6).
Warnings and precautions
Consult your doctor, pharmacist, or nurse before starting to take Sycrest.
Sycrest has not been studied in elderly patients with dementia. However, these patients who are being treated with other types of similar medications may have a higher risk of stroke or death. Sycrest has not been approved for the treatment of elderly patients with dementia and is not recommended for use in this particular group of patients.
Sycrest may cause a drop in blood pressure. In the early stages of treatment, some people may faint, especially if they stand up after being lying down or sitting. It usually goes away on its own, but if it does not, tell your doctor, as you may need a dose adjustment.
Asenapine may cause drowsiness, sudden drops in blood pressure when standing up, dizziness, and changes in your ability to move and balance, which can lead to falls and, consequently, fractures or other traumatic injuries. Patients at risk of falls should be evaluated before prescribing asenapine.
Tell your doctor immediately if you experience
- involuntary rhythmic movements of the tongue, mouth, and face. It may be necessary to discontinue treatment with Sycrest.
- fever, intense muscle stiffness, sweating, or decreased level of consciousness (a disorder called "neuroleptic malignant syndrome"). You may need immediate medical treatment.
Before taking Sycrest, check with your doctor or pharmacist:
- if you have ever been diagnosed with a disease whose symptoms are high body temperature and muscle stiffness (also known as neuroleptic malignant syndrome).
- if you have ever experienced abnormal movements of the tongue or face (tardive dyskinesia).
You should be aware that both diseases can be caused by this type of medication.
- if you have a heart disease or are being treated for a heart disease that may make you prone to low blood pressure
- if you have diabetes or are prone to diabetes
- if you have Parkinson's disease or dementia
- if you have epilepsy (seizures)
- if you experience difficulty swallowing (dysphagia)
- if you have severe liver problems. If you have them, you should not take Sycrest
- if you have a problem controlling body temperature
- if you have thoughts of suicide
- if you have abnormally high levels of prolactin in the blood (hyperprolactinemia)
Make sure to tell your doctor if you are in any of these circumstances so that they can adjust your dose or monitor you for some time. Also, consult your doctor immediately if you develop any of these conditions or if they worsen while you are using Sycrest.
Children and adolescents
The use of Sycrest is not recommended in patients under 18 years of age.
Other medications and Sycrest
Tell your doctor or pharmacist if you are taking, have recently taken, or may need to take any other medication. Some medications can reduce or increase the effect of Sycrest.
If you are taking other medications, Sycrest should be taken last.
You should tell your doctor if you are taking antidepressant medications (specifically fluvoxamine, paroxetine, or fluoxetine), as it may be necessary to change your dose of Sycrest or your dose of antidepressant.
You should tell your doctor if you are taking medications for Parkinson's disease (such as levodopa), as this medication may make them less effective.
Sycrest acts mainly in the brain, so there may be interference with other medications (or with alcohol) that also act in the brain due to a sum of effects on brain function.
Sycrest may decrease blood pressure, so caution should be exercised when Sycrest is taken with other medications that decrease blood pressure.
Taking Sycrest with food, drinks, and alcohol
Do not eat or drink for 10 minutes after taking this medication.
You should avoid drinking alcohol when taking this medication.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medication.
Do not take Sycrest while pregnant, unless your doctor tells you to. If you are taking this medication and become pregnant or plan to become pregnant, consult your doctor as soon as possible to determine if you can continue taking Sycrest.
The following symptoms may occur in newborn babies of mothers who have been treated with Sycrest in the last trimester of pregnancy (last three months of pregnancy): tremors, stiffness and/or muscle weakness, drowsiness, agitation, breathing problems, and difficulty feeding. If your baby develops any of these symptoms, you should contact your doctor.
Do not breastfeed your baby while taking Sycrest.
Driving and using machines
Sycrest may cause drowsiness or sedation. Therefore, make sure your concentration and alertness are not affected before driving or using machines.
3. How to take Sycrest
Follow the administration instructions of this medication exactly as indicated by your doctor or pharmacist. If in doubt, consult your doctor or pharmacist again.
The recommended dose is one 5 or 10 mg sublingual tablet twice a day. One dose should be taken in the morning and another dose should be taken at night.
Instructions for use
Sycrest is for sublingual use.
Sycrest is not recommended if you are unable to take the tablet as described below. If you are unable to take this medication as described below, the treatment may not be effective for you.
- Do not remove the sublingual tablet from the blister until the time of intake.
- Have dry hands when touching the tablet.
- Do not press the tablet against the blister. Do not cut or break the blister.
- Peel off the colored tab (Figure 1).
- Remove the tablet carefully (Figure 2). Do not crush the tablet.
- To ensure optimal absorption, place the tablet under the tongue and wait until it dissolves completely (Figure 3). The tablet will dissolve in saliva in a few seconds.
- Do not swallow or chew the tablet.
- Do not eat or drink for 10 minutes after taking the tablet.
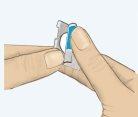
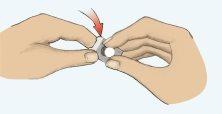
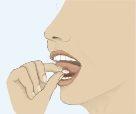
Figure 1 Figure 2 Figure 3
If you take more Sycrest than you should
If you take too much Sycrest, contact a doctor immediately. Bring the medication with you. In case of overdose, you may feel drowsy or tired, have abnormal body movements, problems standing or walking, feel dizzy due to a drop in blood pressure, and feel agitated and confused.
If you forget to take Sycrest
Do not take a double dose to make up for forgotten doses. If you forget to take a dose, take your next dose as usual. If you forget to take two or more doses, consult your doctor or pharmacist.
If you stop taking Sycrest
If you stop taking Sycrest, you will lose the effects of this medication. You should not stop taking this medication unless your doctor tells you to, as your symptoms may recur.
If you have any other questions about the use of this medication, ask your doctor or pharmacist.
4. Possible side effects
Like all medications, this medication can cause side effects, although not everyone gets them.
Serious side effects have been reported with this medication. Seek medical attention immediately if you have any of the following symptoms:
- allergic reactions (usually involving a combination of effects such as difficulty breathing or swallowing, swelling of the face, lips, tongue, or throat, skin rash, itching, and increased heart rate.)
- sudden increase in body temperature, with sweating, rapid pulse, severe muscle stiffness, confusion, and variable blood pressure, which can lead to coma
- seizures, attacks, or crises
- fainting
- falls can occur as a result of one or more side effects such as drowsiness, sudden drops in blood pressure when standing up, dizziness, and changes in your ability to move and balance.
Tell your doctor immediately if you have:
- signs of increased blood sugar levels such as thirst, hunger, or excessive urination, weakness, or worsening of diabetes
- serpentine movements of the tongue, or other uncontrolled movements of the tongue, mouth, cheeks, jaw that can progress to the arms and legs
Other side effects reported with this medication are:
Very common side effects(may affect more than 1 in 10 people)
- anxiety
- drowsiness
Common side effects(may affect up to 1 in 10 people)
- weight gain
- increased appetite
- slow or sustained muscle contractions
- restlessness
- involuntary muscle contractions
- slow movements, tremors
- sedation
- dizziness
- nausea
- change in taste
- feeling of numbness in the tongue or mouth
- increased saliva (drooling)
- muscle stiffness
- fatigue
- increased liver protein levels
Uncommon side effects(may affect up to 1 in 100 people)
- abnormal muscle movements: a set of symptoms known as extrapyramidal symptoms, which may include one or more of the following: abnormal movements of the muscles, tongue, or jaw, slow or sustained muscle contractions, muscle spasms, tremors (agitation), abnormal eye movements, involuntary muscle contractions, slow movements, or restlessness
- unpleasant sensation in the legs (also known as restless legs syndrome)
- difficulty speaking
- abnormally slow or fast heartbeat
- heart block
- abnormal electrocardiogram (prolonged QT interval)
- drop in blood pressure when standing up
- low blood pressure
- tingling in the tongue or mouth
- swollen or painful tongue
- difficulty swallowing
- ulcers, pain, redness, swelling, and blisters inside the mouth
- sexual dysfunction
- absence of regular menstrual periods
Rare side effects(may affect up to 1 in 1,000 people)
- alteration of white blood cell levels (leukocytes)
- difficulty focusing
- blood clots in blood vessels to the lungs, causing chest pain and difficulty breathing
- muscle disease that manifests with unexplained pains
- breast enlargement in men
- spontaneous milk or fluid secretion from the breast
Reporting side effects
If you experience any side effects, consult your doctor, pharmacist, or nurse, even if they are not listed in this package leaflet. You can also report them directly through the national reporting system included in Appendix V. By reporting side effects, you can help provide more information on the safety of this medication.
5. Storage of Sycrest
Keep this medication out of the sight and reach of children.
Do not use this medication after the expiration date shown on the blister and on the packaging. The expiration date is the last day of the month indicated.
Store this medication in its original packaging to protect it from light and moisture.
This medication does not require any special storage temperature.
Medications should not be disposed of through wastewater or household waste. Ask your pharmacist how to dispose of the packaging and any unused medication. This will help protect the environment.
6. Package Contents and Additional Information
Sycrest Composition
- The active ingredient is asenapine.
- Each 5 mg Sycrest sublingual tablet contains 5 mg of asenapine.
- Each 10 mg Sycrest sublingual tablet contains 10 mg of asenapine.
- The exact amount is shown on your Sycrest packaging.
- The other ingredients are gelatin and mannitol (E-421).
Product Appearance and Package Contents
The 5 mg sublingual tablets are round, white to off-white, marked with "5" on one side.
The 10 mg sublingual tablets are round, white to off-white, marked with "10" on one side.
The sublingual tablets are supplied in blister packs containing 10 tablets each. The packages contain 20, 60, or 100 tablets.
Only some package sizes may be marketed.
Marketing Authorization Holder
N.V. Organon
Kloosterstraat 6
NL-5349 AB Oss
Netherlands
Manufacturer
Organon Heist bv
Industriepark 30
2220 Heist-op-den-Berg, Belgium
You can request more information about this medication by contacting the local representative of the marketing authorization holder:
Belgium Organon Belgium Tel: 0080066550123 (+32 2 2418100) | Lithuania Organon Pharma B.V. Lithuania branch Tel.: +370 52041693 |
Bulgaria Organon Bulgaria EOOD Tel: +359 2 806 3030 | Luxembourg Organon Belgium Tel: 0080066550123 (+32 2 2418100) |
Czech Republic Organon Czech Republic s.r.o. Tel: +420 233 010 300 | Hungary Organon Hungary Kft. Tel.: +36 1 766 1963 |
Denmark Organon Denmark ApS Tlf: +45 4484 6800 | Malta Organon Pharma B.V., Cyprus branch Tel: +356 2277 8116 |
Germany Organon Healthcare GmbH Tel.: 0800 3384 726 (+49 (0) 89 2040022 10) | Netherlands N.V. Organon Tel: 00800 66550123 (+32 2 2418100) |
Estonia Organon Pharma B.V. Estonian branch Tel: +372 66 61 300 | Norway Organon Norway AS Tlf: +47 24 14 56 60 |
Greece BIANEΞ Α.Ε. Tel: +30 210 80091 11 | Austria Organon Healthcare GmbH Tel: 0800 3384 726 (+49 (0) 89 2040022 10) |
Spain Organon Salud, S.L. Tel: +34 91 591 12 79 | Poland Organon Polska Sp. z o.o. Tel.: +48 22 105 50 01 |
France Organon France Tel: +33 (0) 1 57 77 32 00 | Portugal Organon Portugal, Sociedade Unipessoal Lda. Tel: +351 218705500 |
Croatia Organon Pharma d.o.o. Tel: +385 1 638 4530 | Romania Organon Biosciences S.R.L. Tel: +40 21 527 29 90 |
Ireland Organon Pharma (Ireland) Limited Tel: +353 15828260 | Slovenia Organon Pharma B.V., Oss, Ljubljana branch Tel: +386 1 300 10 80 |
Iceland Vistor hf. Tel: +354 535 7000 | Slovakia Organon Slovakia s.r.o. Tel: +421 2 44 88 98 88 |
Italy Organon Italia S.r.l. Tel: +39 06 90259059 | Finland Organon Finland Oy Tel: +358 (0) 29 170 3520 |
Cyprus Organon Pharma B.V., Cyprus branch Tel: +357 22866730 | Sweden Organon Sweden AB Tel: +46 8 502 597 00 |
Latvia Organon Pharma B.V. branch in Latvia Tel: +371 66968876 | United Kingdom (Northern Ireland) Organon Pharma (UK) Limited Tel: +44 (0) 208 159 3593 |
Date of the Last Revision of this Leaflet:{month/YYYY}.
Detailed information on this medication is available on the European Medicines Agency website: http://www.ema.europa.eu.
- Country of registration
- Average pharmacy price156.32 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to SYCREST 5 mg SUBLINGUAL TABLETSDosage form: SUBLINGUAL TABLET, 10 mgActive substance: asenapineManufacturer: Organon N.V.Prescription requiredDosage form: PULMONARY INHALATION, 9.1 mgActive substance: loxapineManufacturer: Ferrer Internacional S.A.Prescription requiredDosage form: PULMONARY INHALATION, 9.1 mgActive substance: loxapineManufacturer: Ferrer Internacional S.A.Prescription required
Online doctors for SYCREST 5 mg SUBLINGUAL TABLETS
Discuss questions about SYCREST 5 mg SUBLINGUAL TABLETS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions











