
SYCREST 10 mg SUBLINGUAL TABLETS

How to use SYCREST 10 mg SUBLINGUAL TABLETS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Sycrest 5mg sublingual tablets
Sycrest 10mg sublingual tablets
asenapine
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Sycrest and what is it used for
- What you need to know before you take Sycrest
- How to take Sycrest
- Possible side effects
- Storing Sycrest
- Package contents and further information
1. What is Sycrest and what is it used for
Sycrest contains the active substance asenapine. This medicine belongs to a group of medicines called antipsychotics. Sycrest is used to treat moderate to severe manic episodes associated with bipolar I disorder in adults. Antipsychotic medicines affect the chemicals in the brain that enable communication between brain cells (neurotransmitters). Brain diseases, such as bipolar I disorder, may be due to an imbalance of certain brain chemicals, such as dopamine and serotonin, and these imbalances can cause some of the symptoms you are experiencing. It is not known exactly how this medicine works, but it is thought to adjust the balance of these chemicals.
Manic episodes associated with bipolar I disorder are an illness with symptoms such as feeling well, having excessive energy, needing to sleep much less than usual, talking very quickly with many ideas, and sometimes great irritability.
2. What you need to know before you take Sycrest
Do not take Sycrest
If you are allergic to asenapine or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Talk to your doctor, pharmacist, or nurse before you start taking Sycrest.
Sycrest has not been studied in elderly patients with dementia. However, these patients who are being treated with other types of similar medicines may have a higher risk of stroke or death. Sycrest has not been approved for the treatment of elderly patients with dementia and is not recommended for use in this particular group of patients.
Sycrest may cause a drop in blood pressure. In the early stages of treatment, some people may faint, especially when standing up after lying down or sitting. It usually goes away on its own, but if it does not, tell your doctor, as you may need a dose adjustment.
Asenapine may cause drowsiness, sudden drops in blood pressure when standing up, dizziness, and changes in your ability to move and balance, which can lead to falls and, consequently, fractures or other traumatic injuries. Patients at risk of falls should be assessed before asenapine is prescribed.
Tell your doctor immediately if you experience
- rhythmic involuntary movements of the tongue, mouth, and face. It may be necessary to stop treatment with Sycrest.
- fever, intense muscle stiffness, sweating, or decreased level of consciousness (a disorder called "neuroleptic malignant syndrome"). You may need immediate medical treatment.
Before taking Sycrest, check with your doctor or pharmacist:
- if you have ever been diagnosed with a disease whose symptoms are high body temperature and muscle stiffness (also known as neuroleptic malignant syndrome).
- if you have ever experienced abnormal movements of the tongue or face (tardive dyskinesia).
You should be aware that both diseases can be caused by this type of medicine.
- if you have a heart disease or are being treated for a heart disease that may make you prone to having low blood pressure
- if you have diabetes or are prone to diabetes
- if you have Parkinson's disease or dementia
- if you have epilepsy (seizures)
- if you experience difficulty swallowing (dysphagia)
- if you have severe liver problems. If you have them, you should not take Sycrest
- if you have a problem controlling body temperature
- if you have thoughts of suicide
- if you have abnormally high levels of prolactin in the blood (hyperprolactinemia)
Make sure to tell your doctor if you are in any of these circumstances so that he/she can adjust your dose or monitor you for some time. Also, consult your doctor immediately if you develop any of these conditions or if they worsen while you are using Sycrest.
Children and adolescents
The use of Sycrest is not recommended in patients under 18 years of age.
Other medicines and Sycrest
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines. Some medicines may reduce or increase the effect of Sycrest.
If you are taking other medicines, Sycrest should be taken last.
You should tell your doctor if you are taking antidepressant medicines (specifically fluvoxamine, paroxetine, or fluoxetine), as it may be necessary to change your dose of Sycrest or your dose of antidepressant.
You should tell your doctor if you are taking medicines for Parkinson's disease (such as levodopa), as this medicine may make them less effective.
Sycrest acts mainly in the brain, so there may be interference with other medicines (or with alcohol) that also act in the brain due to a sum of effects on brain function.
Sycrest may decrease blood pressure, so caution should be exercised when Sycrest is taken with other medicines that decrease blood pressure.
Taking Sycrest with food, drinks, and alcohol
Do not eat or drink for 10 minutes after taking this medicine.
You should avoid drinking alcohol when taking this medicine.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
Do not take Sycrest while you are pregnant, unless your doctor tells you to. If you are taking this medicine and become pregnant or plan to become pregnant, consult your doctor as soon as possible to find out if you can continue taking Sycrest.
The following symptoms may occur in newborn babies of mothers who have been treated with Sycrest in the last trimester of pregnancy (last three months of pregnancy): tremors, stiffness and/or muscle weakness, drowsiness, agitation, breathing problems, and difficulty feeding. If your baby develops any of these symptoms, you should contact your doctor.
Do not breastfeed while you are taking Sycrest.
Driving and using machines
Sycrest may cause drowsiness or sedation. Therefore, make sure your concentration and alertness are not affected before driving or using machines.
3. How to take Sycrest
Follow exactly the instructions of administration of this medicine given by your doctor or pharmacist. In case of doubt, consult your doctor or pharmacist again.
The recommended dose is one 5 or 10 mg sublingual tablet twice a day. One dose should be taken in the morning and another dose should be taken at night.
Instructions for use
Sycrest is for sublingual use.
Sycrest is not recommended if you are not able to take the tablet as described below. If you are not able to take this medicine as described below, the treatment may not be effective for you.
- Do not remove the sublingual tablet from the blister until the moment of taking it.
- Have dry hands when touching the tablet.
- Do not press the tablet against the blister. Do not cut or break the blister.
- Peel off the colored tab (Figure 1).
- Remove the tablet carefully (Figure 2). Do not crush the tablet.
- To ensure optimal absorption, place the tablet under the tongue and wait until it dissolves completely (Figure 3). The tablet will dissolve in saliva in a few seconds.
- Do not swallow or chew the tablet.
- Do not eat or drink for 10 minutes after taking the tablet.
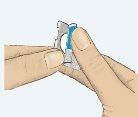
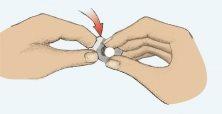
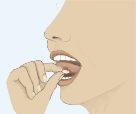
Figure 1 Figure 2 Figure 3
If you take more Sycrest than you should
If you take too much Sycrest, contact a doctor immediately. Bring the medicine with you. In case of overdose, you may feel drowsy or tired, have abnormal body movements, problems standing or walking, feel dizzy due to a drop in blood pressure, and feel agitated and confused.
If you forget to take Sycrest
Do not take a double dose to make up for forgotten doses. If you forget to take a dose, take your next dose as usual. If you forget to take two or more doses, consult your doctor or pharmacist.
If you stop taking Sycrest
If you stop taking Sycrest, you will lose the effects of this medicine. You should not stop taking this medicine unless your doctor tells you to, as your symptoms may come back.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Serious side effects have been reported with this medicine. Seek medical attention immediately if you have any of the following symptoms:
- allergic reactions (usually involving a combination of effects such as difficulty breathing or swallowing, swelling of the face, lips, tongue, or throat, skin rash, itching, and increased heart rate.)
- sudden increase in body temperature, with sweating, rapid pulse, severe muscle stiffness, confusion, and variable blood pressure, which can lead to coma
- seizures, attacks, or crises
- fainting
- falls can occur as a result of one or more side effects such as: drowsiness, sudden drops in blood pressure when standing up, dizziness, and changes in your ability to move and balance.
Tell your doctor immediately if you have:
- signs of high blood sugar levels such as thirst, hunger, or excessive urination, weakness, or worsening of diabetes
- snaking movements of the tongue, or other uncontrolled movements of the tongue, mouth, cheeks, jaw that can progress to the arms and legs
Other side effects reported with this medicine are:
Very common side effects(may affect more than 1 in 10 people)
- anxiety
- drowsiness
Common side effects(may affect up to 1 in 10 people)
- weight gain
- increased appetite
- slow or sustained muscle contractions
- restlessness
- involuntary muscle contractions
- slow movements, tremors
- sedation
- dizziness
- nausea
- change in taste
- feeling of numbness in the tongue or mouth
- increased saliva (drooling)
- muscle stiffness
- fatigue
- increase in liver protein levels
Uncommon side effects(may affect up to 1 in 100 people)
- abnormal movements of the muscles: a set of symptoms known as extrapyramidal symptoms, which may include one or more of the following: abnormal movements of the muscles, tongue, or jaw, slow or sustained muscle contractions, muscle spasms, tremors (agitation), abnormal eye movements, involuntary muscle contractions, slow movements, or restlessness
- unpleasant feeling in the legs (also known as restless legs syndrome)
- difficulty speaking
- abnormally slow or fast heartbeat
- heart block
- abnormal electrocardiogram (prolonged QT interval)
- drop in blood pressure when standing up
- low blood pressure
- tingling in the tongue or mouth
- swollen or painful tongue
- difficulty swallowing
- ulcers, pain, redness, swelling, and blisters inside the mouth
- sexual dysfunction
- absence of regular menstrual periods
Rare side effects(may affect up to 1 in 1,000 people)
- alteration of white blood cell levels (leukocytes)
- difficulty focusing
- blood clots in blood vessels to the lungs, causing chest pain and difficulty breathing
- muscle disease that manifests with unexplained pain
- breast enlargement in men
- spontaneous milk or fluid secretion from the breast
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if it is possible side effects not listed in this leaflet. You can also report side effects directly through the national reporting system included in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storing Sycrest
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the blister and carton. The expiry date is the last day of the month stated.
Store this medicine in the original package to protect it from light and moisture.
This medicine does not require any special storage temperature.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Package Contents and Additional Information
Sycrest Composition
- The active ingredient is asenapine.
- Each 5 mg Sycrest sublingual tablet contains 5 mg of asenapine.
- Each 10 mg Sycrest sublingual tablet contains 10 mg of asenapine.
- The exact amount is shown on your Sycrest packaging.
- The other ingredients are gelatin and mannitol (E-421).
Product Appearance and Package Contents
The 5 mg sublingual tablets are round, white to off-white, marked with "5" on one side.
The 10 mg sublingual tablets are round, white to off-white, marked with "10" on one side.
The sublingual tablets are supplied in peelable blisters, each containing 10 tablets. The packs contain 20, 60 or 100 tablets.
Only some pack sizes may be marketed.
Marketing Authorization Holder
N.V. Organon
Kloosterstraat 6
NL-5349 AB Oss
Netherlands
Manufacturer
Organon Heist bv
Industriepark 30
2220 Heist-op-den-Berg, Belgium
You can request more information about this medicinal product by contacting the local representative of the marketing authorization holder:
Belgium Organon Belgium Tel: 0080066550123 (+32 2 2418100) | Lithuania Organon Pharma B.V. Lithuania branch Tel.: +370 52041693 |
Bulgaria Organon Bulgaria EOOD Tel: +359 2 806 3030 | Luxembourg Organon Belgium Tel: 0080066550123 (+32 2 2418100) |
Czech Republic Organon Czech Republic s.r.o. Tel: +420 233 010 300 | Hungary Organon Hungary Kft. Tel.: +36 1 766 1963 |
Denmark Organon Denmark ApS Tlf: +45 4484 6800 | Malta Organon Pharma B.V., Cyprus branch Tel: +356 2277 8116 |
Germany Organon Healthcare GmbH Tel.: 0800 3384 726 (+49 (0) 89 2040022 10) | Netherlands N.V. Organon Tel: 00800 66550123 (+32 2 2418100) |
Estonia Organon Pharma B.V. Estonian branch Tel: +372 66 61 300 | Norway Organon Norway AS Tlf: +47 24 14 56 60 |
Greece BIANEΞ Α.Ε. Tel: +30 210 80091 11 | Austria Organon Healthcare GmbH Tel: 0800 3384 726 (+49 (0) 89 2040022 10) |
Spain Organon Salud, S.L. Tel: +34 91 591 12 79 | Poland Organon Polska Sp. z o.o. Tel.: +48 22 105 50 01 |
France Organon France Tel: +33 (0) 1 57 77 32 00 | Portugal Organon Portugal, Sociedade Unipessoal Lda. Tel: +351 218705500 |
Croatia Organon Pharma d.o.o. Tel: +385 1 638 4530 | Romania Organon Biosciences S.R.L. Tel: +40 21 527 29 90 |
Ireland Organon Pharma (Ireland) Limited Tel: +353 15828260 | Slovenia Organon Pharma B.V., Oss, Ljubljana branch Tel: +386 1 300 10 80 |
Iceland Vistor hf. Tel: +354 535 7000 | Slovakia Organon Slovakia s.r.o. Tel: +421 2 44 88 98 88 |
Italy Organon Italia S.r.l. Tel: +39 06 90259059 | Finland Organon Finland Oy Tel: +358 (0) 29 170 3520 |
Cyprus Organon Pharma B.V., Cyprus branch Tel: +357 22866730 | Sweden Organon Sweden AB Tel: +46 8 502 597 00 |
Latvia Organon Pharma B.V. branch in Latvia Tel: +371 66968876 | United Kingdom (Northern Ireland) Organon Pharma (UK) Limited Tel: +44 (0) 208 159 3593 |
Date of the last revision of this leaflet:{month/YYYY}.
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu.
- Country of registration
- Average pharmacy price156.32 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to SYCREST 10 mg SUBLINGUAL TABLETSDosage form: SUBLINGUAL TABLET, 5 mgActive substance: asenapineManufacturer: Organon N.V.Prescription requiredDosage form: PULMONARY INHALATION, 9.1 mgActive substance: loxapineManufacturer: Ferrer Internacional S.A.Prescription requiredDosage form: PULMONARY INHALATION, 9.1 mgActive substance: loxapineManufacturer: Ferrer Internacional S.A.Prescription required
Online doctors for SYCREST 10 mg SUBLINGUAL TABLETS
Discuss questions about SYCREST 10 mg SUBLINGUAL TABLETS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions











