
ADASUVE 9.1 mg INHALATION POWDER (SINGLE-DOSE)

How to use ADASUVE 9.1 mg INHALATION POWDER (SINGLE-DOSE)
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
ADASUVE 9.1 mg powder for inhalation (single dose)
loxapine
Read the entire package leaflet carefully before starting to use this medication, as it contains important information for you.
- Keep this package leaflet, as you may need to read it again.
- If you have any questions, consult your doctor or nurse.
- If you experience side effects, consult your doctor or nurse, even if they are side effects not listed in this package leaflet. See section 4.
Contents of the Package Leaflet
- What is ADASUVE and what is it used for
- What you need to know before taking ADASUVE
- How to use ADASUVE
- Possible side effects
- Storage of ADASUVE
- Contents of the pack and further information
1. What is ADASUVE and what is it used for
ADASUVE contains the active substance loxapine, which belongs to a group of medications called antipsychotics. ADASUVE works by blocking certain chemicals in the brain (neurotransmitters), such as dopamine and serotonin, resulting in calming effects and relief from aggressive behavior.
ADASUVE is used to treat acute symptoms of mild to moderate agitation that may occur in adult patients with schizophrenia or bipolar disorder. These are diseases characterized by symptoms such as:
- (Schizophrenia) seeing, hearing, or feeling things that do not exist, distrust, false beliefs, incoherent speech and behavior, and emotional indifference. People with this disease may also feel depressed, guilty, anxious, or tense.
- (Bipolar Disorder) feeling "high" with an excessive amount of energy, needing much less sleep than usual, talking very quickly with many ideas, and sometimes great irritability.
2. What you need to know before taking ADASUVE
Do not use ADASUVE
- if you are allergic to loxapine or amoxapine;
- if you have symptoms of wheezing or shortness of breath;
- if you have lung problems such as asthma or chronic obstructive pulmonary disease (which your doctor may call "COPD").
Warnings and Precautions
Consult your doctor or nurse before starting to use ADASUVE to determine if it is suitable for you.
- ADASUVE may cause narrowing of the airways (bronchospasm) and wheezing, coughing, chest tightness, or shortness of breath. This usually occurs within 25 minutes of administration.
- Neuroleptic Malignant Syndrome (NMS) is a set of symptoms that can occur if you are taking antipsychotics, including ADASUVE. These symptoms can be high fever, muscle stiffness, irregular or rapid heartbeat. NMS can be fatal. Do not use ADASUVE again if you have had NMS.
- Antipsychotic medications like ADASUVE may cause movements that you cannot control, such as facial gestures, sticking out your tongue, hitting or biting your lips, rapid blinking, or moving your legs, arms, or fingers quickly. If this occurs, treatment with ADASUVE should be discontinued.
- ADASUVE will be used with caution in patients who are intoxicated or have delirium.
Before taking ADASUVE, inform your doctor or nurse:
- if you have or have had respiratory problems such as asthma and other chronic lung diseases, such as bronchitis or emphysema.
- if you have or have had heart problems or stroke
- if you have or have had low blood pressure or high blood pressure
- if you have or have had seizures (convulsions)
- if you have or have had glaucoma (increased eye pressure)
- if you have or have had urinary retention (incomplete emptying of the urinary bladder)
- if you have already used ADASUVE and have experienced symptoms of wheezing or shortness of breath
- if you have experienced uncontrollable muscle or eye movements, lack of coordination, constant muscle contraction, or a feeling of restlessness or if you cannot stay still
- if you are an elderly person with dementia (loss of memory and other mental abilities)
Children and Adolescents
ADASUVE is not recommended for use in children and adolescents under 18 years of age.
Using ADASUVE with Other Medications
Inform your doctor if you are taking or have recently taken or may need to take any other medication, including:
- adrenaline
- medications for treating a respiratory problem
- medications that may cause you to have seizures (e.g., clozapine, tricyclics or ISRS, tramadol, mefloquine)
- medications for treating Parkinson's disease
- lorazepam or other central-acting medications (for treating anxiety, depression, pain, or for sleeping) or other medications that cause drowsiness
- illicit drugs
- medications such as fluvoxamine, propranolol, and enoxacin, and other medications that inhibit a liver enzyme called "CYP450 1A2."
- medications for treating schizophrenia, depression, or pain, as you may be at greater risk of having seizures
The use of ADASUVE and adrenaline combined may cause low blood pressure.
Using ADASUVE with Alcohol
Since ADASUVE affects the nervous system, you should avoid consuming alcohol during treatment with ADASUVE.
Pregnancy and Breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor before using this medication. It is recommended that women do not breastfeed for 48 hours after administration of ADASUVE and discard the milk produced during this period. ADASUVE may cause the following symptoms in newborn babies of mothers who have been treated with antipsychotic medications in the last trimester of pregnancy: trembling, stiffness and/or muscle weakness, drowsiness, agitation, breathing problems, and difficulty feeding. If your baby experiences any of these symptoms, you should contact your doctor.
Driving and Using Machines
Do not drive or use tools or machines after taking ADASUVE until you know how it affects you, as the possible side effects of ADASUVE are dizziness, sedation, and drowsiness.
3. How to Use ADASUVE
Always use this medication exactly as instructed by your doctor or nurse. If you are unsure, consult your doctor or nurse again.
The recommended initial dose is 9.1 mg. After 2 hours, your doctor may prescribe a second dose after carefully considering your condition and may reduce the dose to 4.5 mg if your doctor believes this is a more suitable dose for treating your condition.
ADASUVE must be used under the supervision of a doctor or nurse.
ADASUVE is indicated for inhalation use. After the doctor or nurse has prepared ADASUVE, you will be asked to hold the device with your hand, exhale, insert the mouthpiece into your mouth, inhale the medication through the device, and hold your breath for a few seconds.
If You Take More ADASUVE Than You Should
If you think you have taken more ADASUVE than you should, inform your doctor or nurse. Patients who receive more ADASUVE than they should may experience any of the following symptoms: extreme tiredness or drowsiness, breathing problems, low blood pressure, throat irritation, or bad taste in the mouth, uncontrollable muscle or eye movements.
If you have any other questions about using this medication, ask your doctor or nurse.
4. Possible Side Effects
As with all medications, ADASUVE can cause side effects, although not everyone will experience them.
If you experience any of the following side effects, consult your doctor immediately and stop taking the medication:
- respiratory symptoms, such as wheezing, coughing, shortness of breath, or chest tightness, as this may indicate that the medication is irritating the airways (uncommon unless you have asthma or COPD);
- dizziness or fainting, as this may indicate that the medication is lowering your blood pressure (uncommon);
- worsening of agitation or confusion, especially combined with fever or muscle stiffness (rare). These can be associated with a serious condition called Neuroleptic Malignant Syndrome (NMS)
Also, consult your doctor if you experience any of the following side effects that can also occur with other forms of this medication:
Very Common(may affect more than 1 in 10 people): bad taste in the mouth or drowsiness.
Common(may affect up to 1 in 10 people): dizziness, throat irritation, dry mouth, or tiredness
Uncommon(may affect up to 1 in 100 people): uncontrollable muscle or eye movements, lack of coordination, constant muscle contraction, or a feeling of restlessness or if you cannot stay still.
Other additional effects that have been associated with long-term use of loxapine by mouth and may be relevant to ADASUVE include fainting when standing up, increased heart rate, high blood pressure, blurred vision, dry eyes, and decreased urine production.
Reporting Side Effects
If you experience any side effects, consult your doctor or nurse, even if they are side effects not listed in this package leaflet. You can also report side effects directly through the national reporting system included in Appendix V. By reporting side effects, you can help provide more information on the safety of this medication.
5. Storage of ADASUVE
Keep this medication out of sight and reach of children.
Do not use ADASUVE after the expiration date stated on the label. The expiration date is the last day of the month indicated.
Store in the original packaging until use to protect from light and moisture.
Do not use ADASUVE if the bag is open or torn or if there are signs of physical damage to the medication.
Medications should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of the packaging and any unused medication. This will help protect the environment.
6. Contents of the Pack and Further Information
The active substance is loxapine. Each single-dose inhaler contains 10 mg of loxapine and delivers 9.1 mg of loxapine.
Appearance of ADASUVE and Contents of the Pack
ADASUVE 9.1 mg powder for inhalation (single dose) consists of a white plastic, disposable, single-use inhaler containing loxapine. Each inhaler is packaged in a sealed aluminum bag. ADASUVE 9.1 mg is available in a box of 1 or 5 single-dose inhalers.
Marketing Authorization Holder
Ferrer Internacional, S.A.
Gran Vía Carlos III, 94
08028- Barcelona
Spain
Manufacturer
Ferrer Internacional, S.A.
Joan Buscalla, 1-9, 08173 Sant Cugat del Vallès
Barcelona, Spain
You can obtain more information about this medication by contacting the local representative of the marketing authorization holder:
Belgium/Belgique/BelgienLithuania
Ferrer Internacional, S.A. AOP Orphan Pharmaceuticals AG LT
Tel: +34 93 600 37 00 Tel: +370 672 12222
BulgariaLuxembourg/Luxemburg
AOP Orphan Pharmaceuticals AG BG Ferrer Internacional, S.A.
Tel: +359 2 489 9222 Tel: +34 93 600 37 00
Czech RepublicHungary
AOP Orphan Pharmaceuticals AG CZ AOP Orphan Pharmaceuticals AG HU
Tel: +420 251 512 947 Tel: +36 1 3192633
DenmarkMalta
Ferrer Internacional, S.A. Ferrer Internacional, S.A.
Tel: +34 93 600 37 00 Tel: +34 93 600 37 00
GermanyNetherlands
Ferrer Deutschland GmbH Ferrer Internacional, S.A.
Tel: +49 (0) 2407 502311 0 Tel: +34 93 600 37 00
EstoniaNorway
AOP Orphan Pharmaceuticals AG LT Ferrer Internacional, S.A.
Tel: +370 672 12222 Tel: +34 93 600 37 00
GreeceAustria
Ferrer Galenica S.A. AOP Orphan Pharmaceuticals AG
Tel: +30 210 52 81 700 Tel: +43 1 5037244-0
SpainPoland
Ferrer Farma, S.A. AOP Orphan Pharmaceuticals S.A. PL
Tel: +34 93 600 37 00 Tel: +48 22 5424068
FrancePortugal
Ferrer Internacional, S.A. Ferrer Portugal, S.A.
Tel: +34 93 600 37 00 Tel: +351 214449600
CroatiaRomania
AOP Orphan Pharmaceuticals AG Galenica S.A.
Tel: +43 1 5037244-0 Tel: +30 210 52 81 700
IrelandSlovenia
Ferrer Internacional, S.A. AOP Orphan Pharmaceuticals AG
Tel: +34 93 600 37 00 Tel: +43 1 5037244-0
IcelandSlovakia
Ferrer Internacional, S.A. AOP Orphan Pharmaceuticals AG SK
Tel: +34 93 600 37 00 Tel: +421 31 5502271
Italy Angelini S.p.A. Tel: +39 06 780531 | Finland Ferrer Internacional, S.A. Tel: +34 93 600 37 00 |
Cyprus Thespis Pharmaceutical Ltd Tel: +357 22 67 77 10 | Sweden Ferrer Internacional, S.A. Tel: +34 93 600 37 00 |
Latvia AOP Orphan Pharmaceuticals AG LT Tel: +370 672 12222 | United Kingdom Ferrer Internacional, S.A. Tel: +34 93 600 37 00 |
Date of Last Revision of this Package Leaflet:
Other Sources of Information
Detailed information about this medication is available on the European Medicines Agency website: http://www.ema.europa.eu.
This information is intended only for healthcare professionals:
Read all instructions before using this medication. See the SPC for more information.
Familiarize yourself with ADASUVE:The following images show the important elements of ADASUVE.
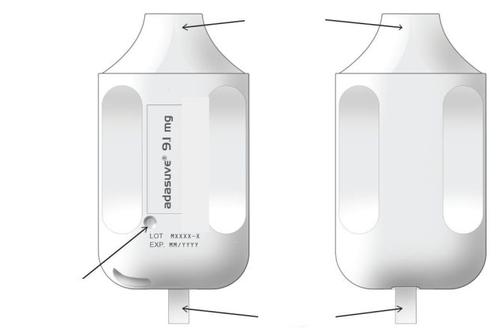 mouthpiece
mouthpiece
powder for inhalation, loxapine |
indicator light
tab
- ADASUVE is packaged in a sealed bag.
- When ADASUVE is removed from the bag, the indicator light is off.
- The indicator light turns on (green) when the tab is removed. The inhaler is ready for use.
- The indicator light turns off automatically again when the medication has been inhaled. Read the following 5 steps before administering ADASUVE to a patient.
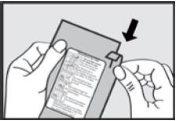
1. Open the bag
Do not open the bag until you are ready to use it.Tear the aluminum bag and remove the inhaler from its packaging.
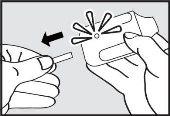
- Pull the tab.
Pull the plastic tab from the back of the inhaler. The green light will turn on, indicating that the inhaler is ready for use.
Use it within the first 15 minutes after removing the tab (or until the green light turns off) to avoid automatic deactivation of the inhaler.
Instruct the patient to:
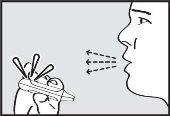
- Exhale
Hold the inhaler away from the mouth and exhale completely to empty the lungs.
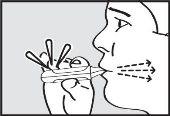
- Inhale
Inhale through the mouthpiece with a deep and continuous inspiration.
IMPORTANT: Check that the green light turns off after inhalation.
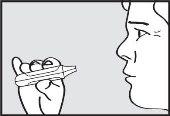
- Hold your breath
Remove the mouthpiece from the mouth and hold your breath for a few seconds.
NOTE: If the green light remains on after the patient has inhaled, instruct the patient to repeat steps 3 to 5.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ADASUVE 9.1 mg INHALATION POWDER (SINGLE-DOSE)Dosage form: PULMONARY INHALATION, 9.1 mgActive substance: loxapineManufacturer: Ferrer Internacional S.A.Prescription requiredDosage form: TABLET, 100 mgActive substance: clozapineManufacturer: Aristo Pharma GmbhPrescription requiredDosage form: TABLET, 200 mgActive substance: clozapineManufacturer: Aristo Pharma GmbhPrescription required
Online doctors for ADASUVE 9.1 mg INHALATION POWDER (SINGLE-DOSE)
Discuss questions about ADASUVE 9.1 mg INHALATION POWDER (SINGLE-DOSE), including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions











