SUNLENCA 464 mg INJECTABLE SOLUTION

How to use SUNLENCA 464 mg INJECTABLE SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Sunlenca 464 mg Solution for Injection
lenacapavir
This medicine is subject to additional monitoring, which will allow for the quick identification of new safety information. You can help by reporting any side effects you may get. The last section of section 4 will tell you how to report side effects.
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Sunlenca and what is it used for
- What you need to know before you are given Sunlenca
- How Sunlenca is given
- Possible side effects
- Storage of Sunlenca
- Contents of the pack and other information
1. What is Sunlenca and what is it used for
Sunlenca contains the active substance lenacapavir. It is an antiretroviral medicine known as a capsid inhibitor.
Sunlenca is a long-acting medicine and is used in combination with other antiretroviral medicinesto treat human immunodeficiency virus type 1 (HIV-1), the virus that causes acquired immune deficiency syndrome (AIDS).
It is used to treat HIV-1 infection in adults with limited treatment options (for example, when other antiretroviral medicines are not effective enough or are not suitable).
Treatment with Sunlenca in combination with other antiretrovirals reduces the amount of HIV in the body. This will improve the function of the immune system (the body's natural defenses) and reduce the risk of developing illnesses associated with HIV infection.
2. What you need to know before you are given Sunlenca
Do not receive Sunlenca
- If you are allergic to lenacapavir or any of the other ingredients of this medicine (listed in section 6).
- If you are taking any of the following medicines:
- rifampicin, used to treat some bacterial infections such as tuberculosis
- carbamazepine, phenytoin, used to prevent seizures
- St. John's Wort (Hypericum perforatum), a herbal medicine used for depression and anxiety
If you think this applies to you, do not receive Sunlenca and tell your doctor immediately.
Warnings and precautions
Talk to your doctor before using Sunlenca
- Talk to your doctor or pharmacist if you have or have ever had severe liver disease or if tests have shown liver problems. Your doctor will carefully consider whether to treat you with Sunlenca.
While you are using Sunlenca
Once you start using Sunlenca, be aware of:
- Signs of inflammation or infection.
If you notice any of these symptoms, tell your doctor immediately.For more information, see section 4, Possible side effects.
Regular appointments are important
It is important that you attend your scheduled appointmentsto receive the Sunlenca injection to control HIV infection and prevent your disease from getting worse. Talk to your doctor if you are considering stopping treatment. If there is a delay in giving you your injection or if you stop receiving Sunlenca, you will need to take other medicines to treat HIV infection and reduce the risk of developing viral resistance.
Children and adolescents
Do not give this medicine to children under 18 years of age. The use of Sunlenca in patients under 18 years of age has not been studied yet, so it is not known how safe and effective the medicine is in this age group.
Other medicines and Sunlenca
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines. Sunlenca may interact with other medicines. This can prevent Sunlenca or other medicines from working properly or can increase the risk of side effects. In some cases, your doctor may need to adjust the dose or check your blood levels.
Medicines that must not be taken with Sunlenca:
- rifampicin, used to treat some bacterial infections such as tuberculosis
- carbamazepine, phenytoin, used to prevent seizures
- St. John's Wort (Hypericum perforatum), a herbal medicine used for depression and anxiety
If you are taking any of these medicines, do not receive Sunlenca injection and tell your doctor immediately.
Tell your doctor especially if you are taking:
- antibiotics that contain:
- rifabutin
- antiepileptics, used to treat epilepsy and prevent seizures (convulsions), that contain:
- oxcarbazepine or phenobarbital
- medicines used to treat HIV, that contain:
- atazanavir/cobicistat, efavirenz, nevirapine, tipranavir/ritonavir or etravirine
- medicines used to treat migraine headaches, that contain:
- dihydroergotamine or ergotamine
- medicines used to treat impotence and pulmonary hypertension, that contain:
- sildenafil or tadalafil
- medicines used to treat impotence, that contain:
- vardenafil
- corticosteroids (also known as “steroids”) given by mouth or by injection, used to treat allergies, inflammatory bowel diseases and other diseases that involve inflammation in the body, that contain:
- dexamethasone or hydrocortisone/cortisone
- medicines used to lower cholesterol, that contain:
- lovastatin or simvastatin
- antiarrhythmics used to treat heart problems, that contain:
- digoxin
- medicines used to help you sleep, that contain:
- midazolam or triazolam
- anticoagulants used to prevent and treat blood clots, that contain:
- rivaroxaban, dabigatran or edoxaban
Tell your doctor if you are taking any of these medicinesor if you start taking any of these medicines during treatment with Sunlenca. Do not stop your treatment without talking to your doctor.
Sunlenca is a long-acting medicine. You should know that if you decide to stop or change your treatment to another one after talking to your doctor, low levels of lenacapavir (the active substance of Sunlenca) may remain in your body for several months after your last injection. It is not expected that the presence of these low levels will affect other antiretroviral medicines you may take later to treat HIV infection. However, the presence of low levels of lenacapavir in your body may affect other medicines you take in the 9 months following your last Sunlenca injection. Ask your doctor if these medicines are safe for you after stopping treatment with Sunlenca.
Pregnancy and breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
As a precaution, you should avoid using Sunlenca during pregnancy unless your doctor tells you otherwise.
Women with HIV-1 infection should not breast-feed their babies, as HIV-1 can be passed to the baby through breast milk. If you are breast-feeding or think you may want to breast-feed, talk to your doctor as soon as possible.
Driving and using machines
Sunlenca is not expected to affect your ability to drive or use machines.
Sunlenca contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per injection; this is, essentially “sodium-free”.
3. How Sunlenca is given
Sunlenca is used in combination with other antiretroviral medicinesto treat HIV infection. Your doctor will tell you which other medicines you should take to treat HIV infection and when you should take them.
Treatment with Sunlenca starts with taking tablets by mouth, followed by injections given by your doctor or nurse, as described below.
Talk to your doctor before taking the tablets.The doctor will tell you when to start taking the tablets and when to schedule your appointment to receive the first injections.
Day 1 of treatment:
- Two tablets by mouth. These can be taken with or without food.
Day 2 of treatment:
- Two tablets by mouth. These can be taken with or without food.
Day 8 of treatment:
- One tablet by mouth. This can be taken with or without food.
Day 15 of treatment:
- Two injections in the abdomen (belly) given at the same time by your doctor or nurse.
Every 6 months:
- Two injections in the abdomen given at the same time by your doctor or nurse.
If you are given more Sunlenca injection than you should
Your doctor or nurse will give you this medicine, so it is unlikely that you will be given too much. Tell your doctor or nurse if you are concerned.
If you miss a Sunlenca injection
- It is important that you attend your scheduled appointments every 6 months to receive the Sunlenca injections. This will help control HIV infection and prevent your disease from getting worse.
- If you think you will not be able to attend your appointment to receive the injections, call your doctor as soon as possible to discuss your treatment options.
Read the package leaflet of Sunlenca tablets if you miss a dose or vomit after taking the tablets.
If you stop treatment with Sunlenca
Do not stop treatment with Sunlenca without talking to your doctor. Continue treatment with Sunlenca injections as long as your doctor recommends. Stopping Sunlenca may seriously affect the effectiveness of future treatments for HIV.
Talk to your doctor if you want to stop treatment with Sunlenca injections.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Serious side effects: tell a doctor immediately
- Any sign of inflammation or infection. In some patients with advanced HIV infection (AIDS) and a history of opportunistic infections (infections that occur in people with a weakened immune system), signs and symptoms of inflammation due to previous infections may occur shortly after starting HIV treatment. These symptoms are thought to be due to an improvement in the body's immune response, which enables it to fight off infections that may have been present without obvious symptoms.
- Autoimmune disorders, where the immune system attacks the body's healthy tissues, can also occur after starting HIV treatment. Autoimmune disorders may occur many months after starting treatment. Be aware of any symptoms of infection or other symptoms, such as:
- muscle weakness
- weakness of the body that starts in the hands and feet and moves towards the trunk
- palpitations, tremors or hyperactivity
If you notice any of these symptoms or any symptoms of inflammation or infection, tell your doctor immediately.
Very common side effects
(may affect more than 1 in 10 people)
- Reactions at the Sunlenca injection site.
Symptoms may include:
- pain and discomfort
- a lump or hardened mass
- inflammatory reaction such as redness, itching and swelling
Common side effects
(may affect up to 1 in 10 people)
- Nausea
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist, even if they are not listed in this leaflet. You can also report side effects directly via the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Sunlenca
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the vial label and carton after {EXP}. The expiry date is the last day of the month shown.
This medicine does not require any special storage conditions. Keep the vial in the original packaging to protect it from light.
6. Container Contents and Additional Information
Sunlenca Composition
The active ingredient is lenacapavir. Each single-dose vial contains 463.5 mg of lenacapavir.
The other components are
Macrogol (E1521), water for injectable preparations.
Appearance of Sunlenca and Container Contents
Sunlenca injectable solution is a clear, yellow to brown solution without visible particles. Sunlenca is presented in two glass vials containing 1.5 ml of injectable solution each. These vials are included in an administration kit that also contains 2 vial access devices (a device that will allow your doctor or nurse to extract Sunlenca from the vial), 2 disposable syringes, and 2 injection needles.
Marketing Authorization Holder
Gilead Sciences Ireland UC
Carrigtohill
County Cork, T45 DP77
Ireland
Manufacturer
Gilead Sciences Ireland UC
IDA Business & Technology Park
Carrigtohill
County Cork
Ireland
You can request more information about this medication by contacting the local representative of the marketing authorization holder:
Belgium Gilead Sciences Belgium SRL-BV Tel: + 32 (0) 24 01 35 50 | Lithuania Gilead Sciences Poland Sp. z o.o. Tel.: + 48 22 262 8702 |
Greece Gilead Sciences Ireland UC Tel: + 353 (0) 1 686 1888 | Luxembourg Gilead Sciences Belgium SRL-BV Tel: + 32 (0) 24 01 35 50 |
Czech Republic Gilead Sciences s.r.o. Tel: + 420 910 871 986 | Hungary Gilead Sciences Ireland UC Tel.: + 353 (0) 1 686 1888 |
Denmark Gilead Sciences Sweden AB Tlf: + 46 (0) 8 5057 1849 | Malta Gilead Sciences Ireland UC Tel: + 353 (0) 1 686 1888 |
Germany Gilead Sciences GmbH Tel: + 49 (0) 89 899890-0 | Netherlands Gilead Sciences Netherlands B.V. Tel: + 31 (0) 20 718 36 98 |
Estonia Gilead Sciences Poland Sp. z o.o. Tel.: +48 22 262 8702 | Norway Gilead Sciences Sweden AB Tlf: + 46 (0) 8 5057 1849 |
Greece Gilead Sciences Ελλάς Μ.ΕΠΕ. Tel: + 30 210 8930 100 | Austria Gilead Sciences GesmbH Tel: + 43 1 260 830 |
Spain Gilead Sciences, S.L. Tel: + 34 91 378 98 30 | Poland Gilead Sciences Poland Sp. z o.o. Tel.: + 48 22 262 8702 |
France Gilead Sciences Tel: + 33 (0) 1 46 09 41 00 | Portugal Gilead Sciences, Lda. Tel: + 351 21 7928790 |
Croatia Gilead Sciences Ireland UC Tel: + 353 (0) 1 686 1888 | Romania Gilead Sciences (GSR) S.R.L. Tel: + 40 31 631 18 00 |
Ireland Gilead Sciences Ireland UC Tel: + 353 (0) 214 825 999 | Slovenia Gilead Sciences Ireland UC Tel: + 353 (0) 1 686 1888 |
Iceland Gilead Sciences Sweden AB Sími: + 46 (0) 8 5057 1849 | Slovakia Gilead Sciences Slovakia s.r.o. Tel: + 421 232 121 210 |
Italy Gilead Sciences S.r.l. Tel: + 39 02 439201 | Finland Gilead Sciences Sweden AB Tel: + 46 (0) 8 5057 1849 |
Cyprus Gilead Sciences Ελλάς Μ.ΕΠΕ. Tel: + 30 210 8930 100 | Sweden Gilead Sciences Sweden AB Tel: + 46 (0) 8 5057 1849 |
Latvia Gilead Sciences Poland Sp. z o.o. Tel.: + 48 22 262 8702 | United Kingdom (Northern Ireland) Gilead Sciences Ireland UC Tel: + 44 (0) 8000 113 700 |
Date of Last Revision of this Prospectus:
Detailed information on this medication is available on the European Medicines Agency website: http://www.ema.europa.eu.
-----------------------------------------------------------------------------
This information is intended solely for healthcare professionals:
Instructions for Use - Sunlenca 464 mg Injectable Solution
The Kit Contains
2 vials |
|
2 vial access devices |
|
2 syringes |
|
2 injection needles |
|
All components are for single use.
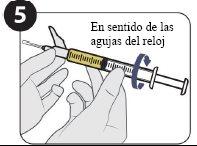
Two 1.5 ml injectionsare required for a complete dose. It is necessary to use the vial access device.
Check that:
- The vial contains a yellow to brown solution without visible particles
- The contents are not damaged
- The medication has not expired
| |
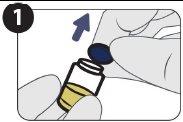
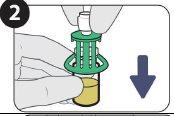
| Remove the cap. |
| Clean the vial seal with an alcohol swab. |
| |
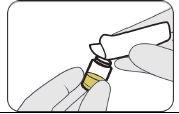
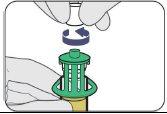
| Push down. |
| Turn to remove. |
| |
|
|
| |
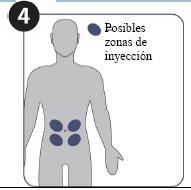
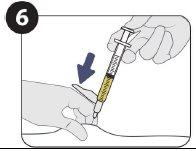
| Possible injection sites (at a minimum of 5 cm from the navel) |
| |
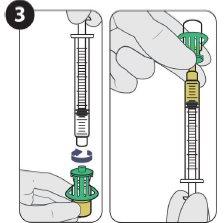
| Attach the injection needle and fill 1.5 ml. |
| |
| Inject 1.5 ml of Sunlenca subcutaneously |
| |
| Repeat the steps for the 2nd injection in a new injection site. |
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to SUNLENCA 464 mg INJECTABLE SOLUTIONDosage form: TABLET, 300 mgActive substance: lenacapavirManufacturer: Gilead Sciences Ireland Unlimited CompanyPrescription requiredDosage form: TABLET, 150 mgActive substance: maravirocManufacturer: Viiv Healthcare B.V.Prescription requiredDosage form: TABLET, 300 mgActive substance: maravirocManufacturer: Viiv Healthcare B.V.Prescription required
Online doctors for SUNLENCA 464 mg INJECTABLE SOLUTION
Discuss questions about SUNLENCA 464 mg INJECTABLE SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions