
STEQEYMA 90 mg SOLUTION FOR INJECTION IN PRE-FILLED SYRINGE

How to use STEQEYMA 90 mg SOLUTION FOR INJECTION IN PRE-FILLED SYRINGE
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Steqeyma 90 mg solution for injection in pre-filled syringe
ustekinumab
This medicine is subject to additional monitoring, which will allow for quick identification of new safety information. You can help by reporting any side effects you may get. The last section of this leaflet includes information on how to report side effects.
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
This leaflet has been written for the person taking the medicine. If you are the parent or carer of a child who will be taking Steqeyma, please read this information carefully.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Steqeyma and what is it used for
- What you need to know before you use Steqeyma
- How to use Steqeyma
- Possible side effects
- Storing Steqeyma
- Contents of the pack and other information
1. What is Steqeyma and what is it used for
1.
Steqeyma contains the active substance “ustekinumab”, a monoclonal antibody. Monoclonal antibodies are proteins that identify and bind to specific proteins in the body.
Steqeyma belongs to a group of medicines called “immunosuppressants”. These medicines work by weakening part of the immune system.
What Steqeyma is used for
Steqeyma is used to treat the following inflammatory diseases:
- Plaque psoriasis - in adults and children aged 6 years and older
- Psoriatic arthritis - in adults
- Moderate to severe Crohn's disease - in adults
Plaque Psoriasis
Plaque psoriasis is a skin disease that causes inflammation affecting the skin and nails. Steqeyma reduces inflammation and other signs of the disease.
Steqeyma is used in adults with moderate to severe plaque psoriasis who cannot use cyclosporin, methotrexate, or phototherapy, or where these treatments do not work.
Steqeyma is used in children and adolescents aged 6 years and older with moderate to severe plaque psoriasis who are not able to tolerate phototherapy or other systemic therapies or where these treatments do not work.
Psoriatic Arthritis
Psoriatic arthritis is an inflammatory disease of the joints, usually accompanied by psoriasis. If you have active psoriatic arthritis, you will first receive other medicines. If you do not respond well to these medicines, you may be treated with Steqeyma to:
- Reduce the signs and symptoms of your disease.
- Improve your physical function.
- Reduce damage to your joints.
Crohn's Disease
Crohn's disease is an inflammatory disease of the intestine. If you have Crohn's disease, you will first be given other medicines. If you do not respond adequately or cannot tolerate these medicines, you may be given Steqeyma to reduce the signs and symptoms of your disease.
2. What you need to know before you use Steqeyma
Do not use Steqeyma
- If you are allergic to ustekinumabor any of the other ingredients of this medicine (listed in section 6).
- If you have an active infectionthat your doctor thinks is important.
If you are not sure if any of the above applies to you, talk to your doctor or pharmacist before using Steqeyma.
Warnings and precautions
Talk to your doctor or pharmacist before starting Steqeyma. Your doctor will check how you are before each treatment. Make sure to tell your doctor about any illness you have before each treatment. Your doctor will also ask if you have recently been near someone who might have tuberculosis. Your doctor will examine you and do a test to check for tuberculosis before you use Steqeyma. If your doctor thinks you are at risk of tuberculosis, you may be given medicines to treat it.
Watch for serious side effects
Steqeyma may cause serious side effects, including allergic reactions and infections. You should be aware of certain signs of illness while you are using Steqeyma. See the complete list of these side effects in “Serious side effects” in section 4.
Tell your doctor before using Steqeyma:
- If you have ever had an allergic reaction to ustekinumab.Talk to your doctor if you are not sure.
- If you have ever had any type of cancer– this is because immunosuppressants like Steqeyma weaken part of the immune system. This may increase the risk of having cancer.
- If you have received treatment for psoriasis with other biologics (a medicine made from a biological source and usually given by injection)– the risk of having cancer may be higher.
- If you have any new or changing lesionswithin the area of psoriasis or on intact skin.
- If you have a recent infection.
- If you are taking any other treatment for psoriasis and/or psoriatic arthritis– such as any other immunosuppressant or phototherapy (when your body is treated with a type of ultraviolet light (UV)). These treatments may also weaken part of the immune system. It has not been studied whether these treatments can be used together with ustekinumab. However, it is possible that it may increase the likelihood of diseases related to a weaker immune system.
- If you are receiving or have ever received allergy shots– it is not known if ustekinumab can affect these treatments.
- If you are 65 years of age or older– you are more likely to get infections.
If you are not sure if you have any of these conditions, talk to your doctor or pharmacist before using Steqeyma.
Some patients have experienced lupus-like reactions during treatment with ustekinumab, including cutaneous lupus or lupus-like syndrome. Talk to your doctor immediately if you experience a red, raised, and scaly skin rash, sometimes with a darker border, in areas of the skin exposed to the sun or if they are accompanied by joint pain.
Heart attacks and strokes
In a study in patients with psoriasis treated with ustekinumab, heart attacks and strokes have been observed. Your doctor will regularly check your risk factors for heart disease and stroke to ensure they are being treated properly. Seek medical attention immediately if you experience chest pain, weakness, or unusual sensation on one side of the body, facial paralysis, or abnormalities in speech or vision.
Children and adolescents
Steqeyma is not recommended for use in children under 6 years of age with psoriasis or in children under 18 years of age with psoriatic arthritis or Crohn's disease, as it has not been studied in this age group.
Other medicines, vaccines, and Steqeyma
Tell your doctor or pharmacist:
- If you are using, have recently used, or might use other medicines.
- If you have been vaccinated recently or are going to have a vaccination.Certain types of vaccines (live vaccines) must not be given while you are using Steqeyma.
- If you received Steqeyma during pregnancy, tell your baby's doctor about your treatment with Steqeyma before your baby receives any vaccination, including live vaccines such as the BCG vaccine (used to prevent tuberculosis).Live vaccines are not recommended for your baby in the first six months after birth if you received Steqeyma during pregnancy, unless your baby's doctor recommends otherwise.
Pregnancy and breastfeeding
- It is recommended to avoid using Steqeyma during pregnancy.The effects of ustekinumab in pregnant women are not known. If you are a woman of childbearing age, you are advised to avoid becoming pregnant and use adequate contraceptive measures while you are using Steqeyma and for at least 15 weeks after the last treatment with Steqeyma.
- Tell your doctor if you are pregnant, think you may be pregnant, or plan to become pregnant.
- Ustekinumab may pass into the placenta to the fetus.If you received Steqeyma during pregnancy, your baby may have a higher risk of getting an infection.
- It is important that you inform your baby's doctors and other healthcare professionals if you received Steqeyma during your pregnancy before your baby receives any vaccination.Live vaccines, such as the BCG vaccine (used to prevent tuberculosis), are not recommended for your baby in the first six months after birth if you received Steqeyma during pregnancy, unless your baby's doctor recommends otherwise.
- Ustekinumab may be present in small amounts in breast milk.Tell your doctor if you are breastfeeding or plan to breastfeed. You and your doctor will decide whether you should breastfeed or use Steqeyma. Do not do both.
Driving and using machines
Steqeyma has no or negligible influence on the ability to drive and use machines.
3. How to use Steqeyma
Steqeyma must be used under the guidance and supervision of a doctor with experience in the treatment of the conditions for which Steqeyma is indicated.
Always follow exactly the administration instructions of this medicine given by your doctor. If you are not sure, ask your doctor. Ask your doctor when you should have the injections and about follow-up appointments.
How much Steqeyma is given
Your doctor will decide how much Steqeyma you need to use and the duration of treatment.
Adults aged 18 years and older Psoriasis or psoriatic arthritis
- The recommended starting dose is 45 mg of Steqeyma.Patients who weigh more than 100 kilograms (kg) may start with a dose of 90 mg instead of 45 mg.
- After the initial dose, you will take the next dose 4 weeks later and then every 12 weeks.The following doses are usually the same as the initial dose.
Crohn's Disease
- During treatment, your doctor will give you the first dose of approximately 6 mg/kg of Steqeyma by infusion into a vein in your arm (intravenous infusion).After the initial dose, you will receive the next dose of 90 mg of Steqeyma 8 weeks later and then every 12 weeks, by injection under the skin (“subcutaneously”).
- In some patients, after the first injection under the skin, 90 mg of Steqeyma will be given every 8 weeks.Your doctor will decide when you should receive the next dose.
Children and adolescents aged 6 years and older Psoriasis
There is no Steqeyma formulation for children with plaque psoriasis with a body weight of less than 60 kg, so other products with ustekinumab should be used.
- Your doctor will tell you the correct dose for you, including the amount (volume) of Steqeyma to inject to give the correct dose.The suitable dose for you will depend on your body weight at the time of each dose.
- If you weigh less than 60 kg, there is no Steqeyma formulation available and other products with ustekinumab should be used.
- If you weigh between 60 kg and 100 kg, the recommended dose is 45 mg of Steqeyma.
- If you weigh more than 100 kg, the recommended dose is 90 mg of Steqeyma.
- After the initial dose, you will receive the next dose 4 weeks later, and then every 12 weeks.
How Steqeyma is given
- Steqeyma is given by injection under the skin (“subcutaneously”).At the start of your treatment, medical or nursing staff may inject Steqeyma for you.
- However, you and your doctor may decide that you will inject Steqeyma yourself.In this case, you will be trained on how to inject Steqeyma yourself.
- For instructions on how to inject Steqeyma, see “Administration instructions” at the end of this leaflet.
Talk to your doctor if you have any questions about how to inject yourself.
If you use more Steqeyma than you should
If you have used or been given too much Steqeyma, talk to your doctor or pharmacist immediately. Always carry the medicine box with you, even if it is empty.
If you forget to use Steqeyma
If you miss a dose, talk to your doctor or pharmacist. Do not take a double dose to make up for forgotten doses.
If you stop using Steqeyma
Stopping Steqeyma is not dangerous. However, if you stop using it, your symptoms may come back.
If you have any other questions about using this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Serious side effects
Some patients may have serious side effects that may need urgent treatment.
Allergic reactions – these may need urgent treatment. Contact your doctor or get emergency medical help immediately if you notice any of the following signs.
- Severe allergic reactions (“anaphylaxis”) are rare in people using ustekinumab (may affect up to 1 in 1,000 people).Signs include:
- difficulty breathing and swallowing
- low blood pressure, which can cause dizziness or mild headaches
- swelling of the face, lips, mouth, or throat.
- Common signs of an allergic reaction include skin rash and hives (these may affect up to 1 in 100 people).
In rare cases, allergic reactions in the lungs and inflammation of the lungs have been reported in patients treated with ustekinumab. Tell your doctor immediately if you have symptoms such as cough, difficulty breathing, and fever.
If you have a severe allergic reaction, your doctor may decide that you should not use Steqeyma again.
Infections – these may need urgent treatment. Contact your doctor immediately if you notice any of these signs.
- Nose or throat infections and the common cold are common (may affect up to 1 in 10 people).
- Chest infections are uncommon (may affect up to 1 in 100 people).
- Inflammation of the tissues under the skin (“cellulitis”) is uncommon (may affect up to 1 in 100 people).
- Herpes (a type of painful rash with blisters) is uncommon (may affect up to 1 in 100 people).
Steqeyma may affect your ability to fight infections. Some of these may be serious and be caused by viruses, fungi, bacteria (including tuberculosis), or parasites, and include infections that occur mainly in people with a weakened immune system (opportunistic infections). Opportunistic infections of the brain (encephalitis, meningitis), lungs, and eyes have been reported in patients receiving treatment with ustekinumab.
You should watch for signs of infection while you are using Steqeyma. These include:
- fever, flu-like symptoms, night sweats, weight loss
- feeling tired or having trouble breathing; cough that does not go away
- having hot, red, and painful skin or having a painful skin rash with blisters
- pain when urinating
- diarrhea
- visual impairment or loss of vision
- headache, stiff neck, sensitivity to light, nausea, or confusion.
Talk to your doctor immediately if you notice any of these signs of infection, as they may be signs of infections such as chest infections, skin infections, herpes, or opportunistic infections that may have serious complications. You should also tell your doctor if you have any infection that does not go away or comes back. Your doctor may decide that you should not use Steqeyma until the infection goes away. Also, talk to your doctor if you have any open cuts or ulcers that may become infected.
Shedding of the skin – the increase in redness and shedding of the skin on a large surface of the body can be symptoms of erythrodermic psoriasis or exfoliative dermatitis, which are serious skin disorders. If you notice any of these symptoms, you should inform your doctor immediately.
Other adverse effects
Frequent adverse effects(may affect up to 1 in 10 people):
- Diarrhea
- Nausea
- Vomiting
- Feeling of fatigue
- Feeling of dizziness
- Headache
- Itching ("pruritus")
- Back, muscle, or joint pain
- Sore throat
- Redness and pain at the injection site
- Sinusitis
Uncommon adverse effects(may affect up to 1 in 100 people):
- Dental infections
- Vaginal yeast infections
- Depression
- Nasal congestion or blockage
- Bleeding, bruising, hardening, swelling, and itching at the injection site
- Feeling weak
- Drooping eyelid and sinking of the muscles on one side of the face ("facial paralysis" or "Bell's palsy"), which is usually temporary
- A change in psoriasis with redness and new small, yellow or white skin blisters, sometimes accompanied by fever (pustular psoriasis).
- Scaling of the skin (skin exfoliation)
- Acne
Rare adverse effects(may affect up to 1 in 1,000 people):
- Redness and shedding of the skin on a large surface of the body, which can cause itching or pain (exfoliative dermatitis). Similar symptoms may develop as a natural change in psoriasis symptoms (erythrodermic psoriasis)
- Inflammation of small blood vessels, which can cause a skin rash with small red or purple bumps, fever, or joint pain (vasculitis)
Very rare adverse effects(may affect up to 1 in 10,000 people)
- Blisters on the skin, which can be red and cause itching and pain (bullous pemphigoid).
- Cutaneous lupus or lupus-like syndrome (red, raised, and scaly skin rash in sun-exposed areas, possibly accompanied by joint pain).
Reporting of adverse effects
If you experience any type of adverse effect, consult your doctor or pharmacist, even if it is a possible adverse effect that is not listed in this leaflet. You can also report them directly through the national reporting system included in Appendix V. By reporting adverse effects, you can contribute to providing more information on the safety of this medication.
5. Storage of Steqeyma
- Keep this medication out of sight and reach of children.
- Store in the refrigerator (between 2 °C and 8 °C). Do not freeze.
- Keep the pre-filled syringe in the outer packaging to protect it from light.
- If necessary, individual pre-filled syringes of Steqeyma can also be stored at room temperature up to 30 °C for a maximum of 31 days in the original carton to protect them from light. Write the date when the pre-filled syringe is first removed from the refrigerator and the date when it should be discarded on the space provided on the outer packaging. The discard date should not exceed the original expiry date printed on the carton. Once a syringe has been stored at room temperature (up to a maximum of 30 °C), it should not be stored in the refrigerator again. Discard the syringe if it is not used within 31 days of storage at room temperature or by the original expiry date, whichever comes first.
- Do not shake the pre-filled syringes of Steqeyma. Prolonged vigorous shaking can damage the product.
Do not use this medication:
- After the expiry date stated on the label and carton after "EXP". The expiry date is the last day of the month indicated.
- If the liquid changes color, is cloudy, or has foreign particles floating in it (see section 6 "Appearance of Steqeyma and package contents").
- If you know or believe that it has been exposed to extreme temperatures (such as accidental heating or freezing).
- If the product has been shaken vigorously.
Steqeyma is for single use. You should discard any unused product left in the syringe. Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of the packaging and any unused medication. This will help protect the environment.
6. Package contents and additional information
Composition of Steqeyma
- The active substance is ustekinumab. Each pre-filled syringe contains 90 mg of ustekinumab in 1 ml.
- The other ingredients are L-histidine, L-histidine monohydrochloride monohydrate, polysorbate 80, sucrose, and water for injectable preparations.
Appearance of Steqeyma and package contents
Steqeyma is a clear or slightly opalescent (with a pearlescent sheen) injectable solution, colorless to pale yellow. The solution may contain a few translucent or white protein particles. It is presented in a pack containing 1 single-dose pre-filled syringe of 1 ml of glass. Each pre-filled syringe contains 90 mg of ustekinumab in 1 ml of injectable solution.
Marketing Authorization Holder
Celltrion Healthcare Hungary Kft. 1062 Budapest
Váci út 1-3. WestEnd Office Building B tower Hungary
Manufacturer
Nuvisan France SARL 2400, Route des Colles 06410, Biot
France
MIDAS Pharma GmbH Rheinstrasse 49
55218 West Ingelheim Am Rhein Rhineland-Palatinate
Germany
Kymos S.L.
Ronda de Can Fatjó 7B Parc Tecnològic del Vallès
08290 Cerdanyola Del Valles Barcelona
Spain
You can request more information about this medication by contacting the local representative of the marketing authorization holder:
Spain
Kern Pharma, S.L.
Tel: +34 93 700 2525
Date of the last revision of this leaflet <{MM/AAAA}>.
Detailed information on this medication is available on the European Medicines Agency website: https://www.ema.europa.eu.
Administration instructions
At the start of treatment, your healthcare professional will help you with the first injection. However, you and your doctor may decide that you can inject Steqeyma yourself. If this happens, you will receive training on how to inject Steqeyma. Talk to your doctor if you have any questions about how to inject yourself.
Important information
- Do notopen the sealed carton until you are ready to use the pre-filled syringe.
- Do notremove the cap until just before administering the injection.
- Do notmix Steqeyma with other injectable liquids.
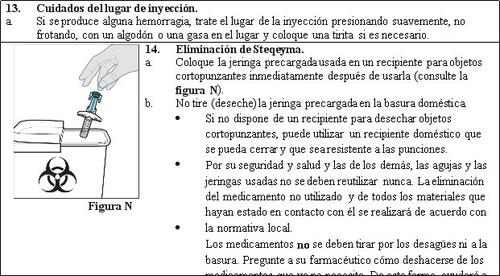
- The pre-filled syringe cannot be reused. Discard the used pre-filled syringe immediately after use in a puncture-resistant container (see step 14. Disposal of Steqeyma).
Storage of Steqeyma
- Keep the pre-filled syringe out of sight and reach of children.It contains small parts.
- Store the pre-filled syringe in the refrigerator between 2 °C and 8 °C. Do notfreeze.
- Keep this medication in its closed packaging to protect it from light.
- If necessary, individual pre-filled syringes of Steqeyma can also be stored at room temperature up to 30 °C for a maximum of 31 days in the original carton to protect them from light.
- Do notshake the pre-filled syringes of Steqeyma. Vigorous shaking can damage the medication.
- Do notuse the medication if it has been shaken vigorously.
- Do notuse the pre-filled syringe if it has been dropped.
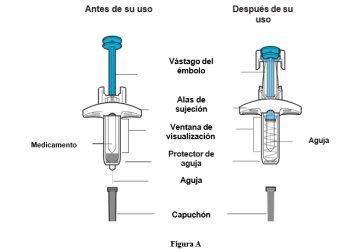
Parts of the Pre-filled Syringe (see Figure A)
Preparation for Injection
|
Not included in the box:
| |
gauze and pre-filled syringe with alcohol swab | ||
| ||
Adhesive bandage Container for sharp objects | ||
Figure B |
| |
Figure C |
| |
30
Figure D |
| |
Figure E |
|
Figure F |
| |
= Self-injection and caregiver Figure G |
| |
Figure H |
Figure H). | |
|
| |
Figure I |
Administration of the Injection
Figure JDo notuse the pre-filled syringe if it falls without the needle cap in place. If this happens, contact your doctor or pharmacist.
|
Figure K |
Figure L |
Figure M |
After the Injection
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to STEQEYMA 90 mg SOLUTION FOR INJECTION IN PRE-FILLED SYRINGEDosage form: INJECTABLE PERFUSION, 130 mgActive substance: ustekinumabManufacturer: Accord Healthcare S.L.U.Prescription requiredDosage form: INJECTABLE, 45 mgActive substance: ustekinumabManufacturer: Accord Healthcare S.L.U.Prescription requiredDosage form: INJECTABLE, 90 mgActive substance: ustekinumabManufacturer: Accord Healthcare S.L.U.Prescription required
Online doctors for STEQEYMA 90 mg SOLUTION FOR INJECTION IN PRE-FILLED SYRINGE
Discuss questions about STEQEYMA 90 mg SOLUTION FOR INJECTION IN PRE-FILLED SYRINGE, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions





 ALCOHOL SWAB
ALCOHOL SWAB Box containing cotton or
Box containing cotton or

 EXP:MM/YYYY
EXP:MM/YYYY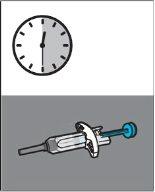 minutes
minutes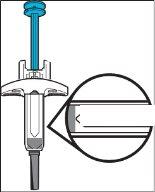 EXP:MM/YYYY
EXP:MM/YYYY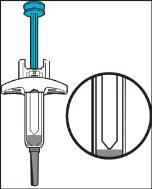
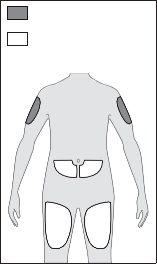 = ONLY caregiver
= ONLY caregiver
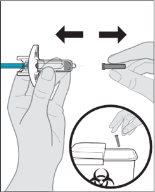 Remove the cap.
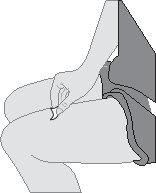
Remove the cap.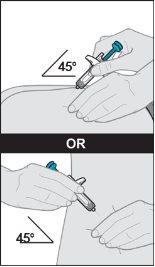 ORneedle completely into the skin fold at a 45-degree angle (see Figure K).
ORneedle completely into the skin fold at a 45-degree angle (see Figure K).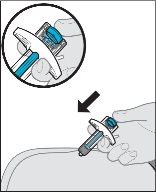 After inserting the needle, release the pinch.
After inserting the needle, release the pinch.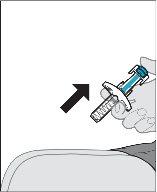 Remove the pre-filled syringe from the injection site.
Remove the pre-filled syringe from the injection site.
