
STELARA 90 mg SOLUTION FOR INJECTION IN PRE-FILLED PEN

How to use STELARA 90 mg SOLUTION FOR INJECTION IN PRE-FILLED PEN
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
STELARA 90 mg Solution for Injection in Pre-filled Pen
Ustekinumab
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
This leaflet has been written for the person taking the medicine.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Stelara and what is it used for
- What you need to know before you use Stelara
- How to use Stelara
- Possible side effects
- Storing Stelara
- Contents of the pack and other information
1. What is Stelara and what is it used for
What is Stelara
Stelara contains the active substance “ustekinumab”, a monoclonal antibody. Monoclonal antibodies are proteins that identify and bind specifically to certain proteins in the body.
Stelara belongs to a group of medicines called “immunosuppressants”. These medicines work by weakening part of the immune system.
What is Stelara used for
Stelara administered with the pre-filled pen is used to treat the following inflammatory diseases:
- Plaque psoriasis – in adults
- Psoriatic arthritis – in adults
- Moderate to severe Crohn's disease – in adults
- Moderate to severe ulcerative colitis – in adults
Plaque Psoriasis
Plaque psoriasis is a skin disease that causes inflammation affecting the skin and nails. Stelara reduces inflammation and other signs of the disease.
Stelara administered with the pre-filled pen is used in adults with moderate to severe plaque psoriasis who cannot use cyclosporin, methotrexate, or phototherapy, or when these treatments have not worked.
Psoriatic Arthritis
Psoriatic arthritis is an inflammatory disease of the joints, usually accompanied by psoriasis. If you have active psoriatic arthritis, you will first receive other medicines. If you do not respond well to these medicines, you may be treated with Stelara to:
- Reduce the signs and symptoms of your disease.
- Improve your physical function.
- Reduce damage to your joints.
Crohn's Disease
Crohn's disease is an inflammatory disease of the intestine. If you have Crohn's disease, you will first be given other medicines. If you do not respond adequately or cannot tolerate these medicines, you may be given Stelara to reduce the signs and symptoms of your disease.
Ulcerative Colitis
Ulcerative colitis is an inflammatory disease of the intestine. If you have ulcerative colitis, you will first be given other medicines. If you do not respond well enough or cannot tolerate these medicines, you may be given Stelara to reduce the signs and symptoms of your disease.
2. What you need to know before you use Stelara
Do not use Stelara
- If you are allergic to ustekinumabor any of the other ingredients of this medicine (listed in section 6).
- If you have an active infectionthat your doctor thinks is important.
If you are not sure if any of the above applies to you, talk to your doctor or pharmacist before using Stelara.
Warnings and precautions
Talk to your doctor or pharmacist before starting Stelara. Your doctor will check how you are before each treatment. Make sure you tell your doctor about any illness you have before each treatment. Your doctor will also ask if you have recently been near someone who might have tuberculosis. Your doctor will examine you and do a test to check for tuberculosis before you use Stelara. If your doctor thinks you are at risk of tuberculosis, they may give you medicines to treat it.
Watch for serious side effects
Stelara may cause serious side effects, including allergic reactions and infections. You should be aware of certain signs of illness while you are using Stelara. See the complete list of these side effects in “Serious side effects” in section 4.
Tell your doctor before using Stelara:
- If you have ever had an allergic reaction to Stelara.Talk to your doctor if you are not sure.
- If you have ever had any type of cancer– this is because immunosuppressants like Stelara weaken part of the immune system. This may increase the risk of having cancer.
- If you have received treatment for psoriasis with other biologics (a medicine made from a biological source and usually given by injection)– the risk of having cancer may be higher.
- If you have had a recent infection.
- If you have any new or changing lesionswithin the area of psoriasis or on intact skin.
- If you have ever had an allergic reaction to latex or to the Stelara injection– the packaging of this medicine contains latex, which may cause serious allergic reactions in people who are sensitive to latex. See “Watch for serious side effects” in section 4 for signs of an allergic reaction.
- If you are taking any other treatment for psoriasis and/or psoriatic arthritis– such as any other immunosuppressant or phototherapy (when your body is treated with a type of ultraviolet light (UV)). These treatments may also weaken part of the immune system. It has not been studied whether these treatments can be used together with Stelara. However, it may increase the likelihood of suffering from diseases related to a weaker immune system.
- If you have received allergy shots– it is not known if Stelara can affect these treatments.
- If you are 65 years of age or older– you are more likely to get infections.
If you are not sure if you have any of these conditions, talk to your doctor or pharmacist before using Stelara.
Some patients have experienced lupus-like reactions during treatment with ustekinumab, including cutaneous lupus or lupus-like syndrome. Talk to your doctor immediately if you experience a red, raised, and scaly skin rash, sometimes with a darker border, in areas of the skin exposed to the sun or if they are accompanied by joint pain.
Heart attacks and strokes
In a study of patients with psoriasis treated with Stelara, heart attacks and strokes have been observed. Your doctor will regularly check your risk factors for heart disease and stroke to ensure they are being treated properly. Seek medical attention immediately if you experience chest pain, weakness, or an unusual feeling on one side of your body, facial paralysis, or abnormalities in speech or vision.
Children and adolescents
Stelara in the pre-filled pen is not recommended for use in children and adolescents under 18 years of age with psoriasis, as it has not been studied in this age group. For children from 6 years of age and adolescents with psoriasis, the pre-filled syringe or vial should be used instead.
Stelara is not recommended for use in children and adolescents under 18 years of age with psoriatic arthritis, Crohn's disease, or ulcerative colitis, as it has not been studied in this age group.
Using Stelara with other medicines, vaccines
Tell your doctor or pharmacist:
- If you are using, have recently used, or might use other medicines.
- If you have been vaccinated recently or are going to have a vaccination.Certain types of vaccines (live vaccines) must not be given while you are using Stelara.
- If you received Stelara during pregnancy, inform your baby's doctor about your treatment with Stelara before your baby receives any vaccination, including live vaccines such as the BCG vaccine (used to prevent tuberculosis).Live vaccines are not recommended for your baby in the first six months after birth if you received Stelara during pregnancy, unless your baby's doctor recommends otherwise.
Pregnancy and breastfeeding
- It is recommended to avoid using Stelara during pregnancy.The effects of Stelara in pregnant women are not known. If you are a woman of childbearing age, you are advised to avoid becoming pregnant and to use adequate contraceptive measures while you are using Stelara and for at least 15 weeks after the last treatment with Stelara.
- Tell your doctor if you are pregnant, think you may be pregnant, or plan to become pregnant.
- Stelara may pass through the placenta to the fetus.If you received Stelara during pregnancy, your baby may have a higher risk of getting an infection.
- It is important that you inform your baby's doctors and other healthcare professionals if you received Stelara during your pregnancy before your baby receives any vaccination.Live vaccines, such as the BCG vaccine (used to prevent tuberculosis), are not recommended for your baby in the first six months after birth if you received Stelara during pregnancy, unless your baby's doctor recommends otherwise.
- Ustekinumab may be excreted in breast milk in very small amounts.Tell your doctor if you are breastfeeding or plan to breastfeed. You and your doctor will decide whether you should breastfeed or use Stelara. Do not do both.
Driving and using machines
Stelara has no or negligible influence on the ability to drive and use machines.
3. How to use Stelara
Stelara must be used under the guidance and supervision of a doctor with experience in the treatment of the conditions for which Stelara is indicated.
Always follow exactly the administration instructions of this medicine given by your doctor. If you are not sure, ask your doctor. Ask your doctor when you should have the injections and about follow-up appointments.
How much Stelara to use
Your doctor will decide how much Stelara you need to use and how long your treatment will last.
Adults from 18 years of age
Psoriasis or psoriatic arthritis
- The recommended starting dose is 45 mg of Stelara.Patients who weigh more than 100 kilograms (kg) may start with a dose of 90 mg instead of 45 mg.
- After the initial dose, the next dose will be given 4 weeks later and then every 12 weeks.The following doses are usually the same as the starting dose.
Crohn's disease or ulcerative colitis
- During treatment, your doctor will give you the first dose of approximately 6 mg/kg of Stelara through a drip in a vein in your arm (intravenous infusion).After the initial dose, you will receive the next dose of 90 mg of Stelara 8 weeks later and then every 12 weeks, through an injection under the skin (“subcutaneously”).
- In some patients, after the first injection under the skin, 90 mg of Stelara will be given every 8 weeks.Your doctor will decide when you should have the next dose.
How Stelara is given
- Stelara is given through an injection under the skin (“subcutaneously”).At the start of your treatment, medical staff may give you the injection.
- However, you and your doctor may decide that you can inject Stelara yourself.In this case, you will be trained on how to inject Stelara yourself.
- For instructions on how to inject Stelara, see “Administration instructions” at the end of this leaflet.
Talk to your doctor if you have any questions about how to inject yourself.
If you use more Stelara than you should
If you have used or been given too much Stelara, talk to your doctor or pharmacist immediately. Always carry the medicine box with you, even if it is empty.
If you forget to use Stelara
If you miss a dose, talk to your doctor or pharmacist. Do not take a double dose to make up for forgotten doses.
If you stop using Stelara
Stopping Stelara is not dangerous. However, if you stop using it, your symptoms may come back.
If you have any other questions about using this medicine, ask your doctor or pharmacist.
4. Possible Adverse Effects
Like all medicines, this medicine can cause adverse effects, although not all people suffer from them.
Severe Adverse Effects
Some patients may have severe adverse effects that may require urgent treatment.
Allergic Reactions – these may require urgent treatment. Contact your doctor or get emergency medical help immediately if you notice any of the following signs.
- Severe allergic reactions ("anaphylaxis") are rare in the population using Stelara (may affect up to 1 in 1,000 people). The signs include:
- difficulty breathing and swallowing
- low blood pressure, which can cause dizziness or mild headaches
- swelling of the face, lips, mouth, or throat.
- Common signs of an allergic reaction include skin rash and hives (these may affect up to 1 in 100 people).
In rare cases, allergic reactions at the lung level and lung inflammation have been reported in patients treated with ustekinumab. Inform your doctor immediately if you have symptoms such as cough, difficulty breathing, and fever.
If you have a severe allergic reaction, your doctor may decide that you should not use Stelara again.
Infections – these may require urgent treatment. Contact your doctor immediately if you notice any of these signs.
- Nose or throat infections and the common cold are frequent (may affect up to 1 in 10 people).
- Chest infections are uncommon (may affect up to 1 in 100 people).
- Inflammation of the tissues under the skin ("cellulitis") is uncommon (may affect up to 1 in 100 people).
- Herpes (a type of painful rash with blisters) is uncommon (may affect up to 1 in 100 people).
Stelara may affect your ability to fight infections. Some of them could become serious and be caused by viruses, fungi, bacteria (including tuberculosis), or parasites, and include infections that occur mainly in people with a weakened immune system (opportunistic infections). Opportunistic infections of the brain (encephalitis, meningitis), lungs, and eyes have been reported in patients receiving treatment with ustekinumab.
You should watch for signs of infection while using Stelara. These include:
- fever, flu-like symptoms, night sweats, weight loss
- feeling tired or having difficulty breathing; cough that does not go away
- having hot, red, and painful skin or having a painful skin rash with blisters
- burning when urinating
- diarrhea
- visual impairment or loss of vision
- headache, neck stiffness, photosensitivity, nausea, or confusion.
Communicate with your doctor immediately if you notice any of these signs of infection, as they may be signs of infections such as chest infections, skin infections, herpes, or opportunistic infections that could have serious complications. You should also inform your doctor if you have any type of infection that does not go away or comes back. Your doctor may decide that you should not use Stelara until the infection goes away. Also, contact your doctor if you have any open cuts or ulcers that could become infected.
Skin Detachment – increased redness and skin detachment over a large area of the body may be symptoms of erythrodermic psoriasis or exfoliative dermatitis, which are serious skin disorders. If you notice any of these symptoms, you should inform your doctor immediately.
Other Adverse Effects
Frequent Adverse Effects(may affect up to 1 in 10 people):
- Diarrhea
- Nausea
- Vomiting
- Feeling tired
- Feeling dizzy
- Headache
- Itching ("pruritus")
- Back, muscle, or joint pain
- Sore throat
- Redness and pain at the injection site
- Sinusitis
Uncommon Adverse Effects(may affect up to 1 in 100 people):
- Dental infections
- Vaginal yeast infections
- Depression
- Nasal congestion or stuffiness
- Bleeding, bruising, hardening, swelling, and itching at the injection site
- Feeling weak
- Drooping eyelid and sinking of the muscles on one side of the face ("facial paralysis" or "Bell's palsy"), which is usually temporary
- A change in psoriasis with redness and new small, yellow, or white skin blisters, sometimes accompanied by fever (pustular psoriasis).
- Skin peeling (skin exfoliation)
- Acne
Rare Adverse Effects(may affect up to 1 in 1,000 people):
- Redness and skin detachment over a large area of the body, which may cause itching or pain (exfoliative dermatitis). Similar symptoms may develop as a natural change in psoriasis symptoms (erythrodermic psoriasis)
- Inflammation of small blood vessels, which may cause a skin rash with small red or purple bumps, fever, or joint pain (vasculitis)
Very Rare Adverse Effects(may affect up to 1 in 10,000 people)
- Blisters on the skin, which may be red and cause itching and pain (bullous pemphigoid).
- Cutaneous lupus or lupus-like syndrome (red, raised, and scaly skin rash in sun-exposed areas, possibly accompanied by joint pain).
Reporting Adverse Effects
If you experience any type of adverse effect, consult your doctor or pharmacist, even if it is a possible adverse effect that is not listed in this leaflet. You can also report them directly through the national reporting system included in Appendix V. By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Storage of Stelara
- Keep this medicine out of the sight and reach of children.
- Store in a refrigerator (2°C and 8°C). Do not freeze.
- Keep the pre-filled pen in the outer packaging to protect it from light.
- If necessary, individual pre-filled Stelara pens can also be stored at room temperature up to 30°C for a maximum single period of up to 30 days, keeping them in their original box to protect them from light. Write the date when the pre-filled pen is first removed from the refrigerator and the date when it should be discarded on the space provided on the outer packaging. The discard date should not exceed the original expiry date printed on the box. Once a syringe or pre-filled pen has been stored at room temperature (up to a maximum of 30°C), it should not be stored in the refrigerator again. Discard the syringe or pre-filled pen if it has not been used within 30 days of storage at room temperature or if it has expired according to the original expiry date, whichever comes first.
- Do not shake Stelara pre-filled pens. Prolonged vigorous shaking may damage the product.
Do not use this medicine:
- After the expiry date stated on the label and carton after "EXP". The expiry date is the last day of the month indicated.
- If the liquid changes color, is cloudy, or has particles floating in it (see section 6 "Appearance of Stelara and package contents").
- If you know or believe that it has been exposed to extreme temperatures (such as accidental heating or freezing).
- If the product has been shaken vigorously.
Stelara is for single use. You should discard any unused product left in the syringe or pre-filled pen. Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of the packaging and medicines you no longer need. This will help protect the environment.
6. Container Contents and Additional Information
Stelara Composition
- The active ingredient is ustekinumab. Each pre-filled syringe contains 90 mg of ustekinumab in 1 ml.
- The other components are L-histidine, L-histidine monohydrate monohydrochloride, polysorbate 80, sucrose, and water for injectable preparations.
Appearance of Stelara and Container Contents
Stelara is a slightly opalescent (with a pearlescent sheen), colorless to light yellow transparent injectable solution. The solution may contain a few translucent or white protein particles. It is presented in a container that contains 1 pre-filled syringe of 1 ml of glass. Each pre-filled syringe contains 90 mg of ustekinumab in 1 ml of injectable solution.
Marketing Authorization Holder
Janssen-Cilag International NV
Turnhoutseweg 30
B-2340 Beerse
Belgium
Manufacturer
Janssen Biologics B.V.
Einsteinweg 101
2333 CB Leiden
Netherlands
You can request more information about this medication by contacting the local representative of the marketing authorization holder:
België/Belgique/Belgien Janssen-Cilag NV Tel/Tél: +32 14 64 94 11 | Lietuva UAB "JOHNSON & JOHNSON" Tel: +370 5 278 68 88 |
| Luxembourg/Luxemburg Janssen-Cilag NV Tél/Tel: +32 14 64 94 11 |
Ceská republika Janssen-Cilag s.r.o. Tel: +420 227 012 227 | Magyarország Janssen-Cilag Kft. Tel.: +36 1 884 2858 |
Danmark Janssen-Cilag A/S Tlf: +45 4594 8282 | Malta AM MANGION LTD Tel: +356 2397 6000 |
Deutschland Janssen-Cilag GmbH Tel: +49 2137 955 955 | Nederland Janssen-Cilag B.V. Tel: +31 76 711 1111 |
Eesti UAB "JOHNSON & JOHNSON" Eesti filiaal Tel: +372 617 7410 | Norge Janssen-Cilag AS Tlf: +47 24 12 65 00 |
| Österreich Janssen-Cilag Pharma GmbH Tel: +43 1 610 300 |
España Janssen-Cilag, S.A. Tel: +34 91 722 81 00 | Polska Janssen-Cilag Polska Sp. z o.o. Tel.: +48 22 237 60 00 |
France Janssen-Cilag Tél: 0 800 25 50 75 / +33 1 55 00 40 03 | Portugal Janssen-Cilag Farmacêutica, Lda. Tel: +351 214 368 600 |
Hrvatska Johnson & Johnson S.E. d.o.o. Tel: +385 1 6610 700 | România Johnson & Johnson România SRL Tel: +40 21 207 1800 |
Ireland Janssen Sciences Ireland UC Tel: 1 800 709 122 | Slovenija Johnson & Johnson d.o.o. Tel: +386 1 401 18 00 |
Ísland Janssen-Cilag AB c/o Vistor hf. Sími: +354 535 7000 | Slovenská republika Johnson & Johnson, s.r.o. Tel: +421 232 408 400 |
Italia Janssen-Cilag SpA Tel: 800.688.777 / +39 02 2510 1 | Suomi/Finland Janssen-Cilag Oy Puh/Tel: +358 207 531 300 |
| Sverige Janssen-Cilag AB Tfn: +46 8 626 50 00 |
Latvija UAB "JOHNSON & JOHNSON" filiale Latvija Tel: +371 678 93561 | United Kingdom (Northern Ireland) Janssen Sciences Ireland UC Tel: +44 1 494 567 444 |
Date of Last Revision of this Leaflet
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu/.
Instructions for Use
Stelara
(ustekinumab)
injection, for subcutaneous use
Pre-filled Syringe
These instructions for use contain information on how to inject Stelara.
Important
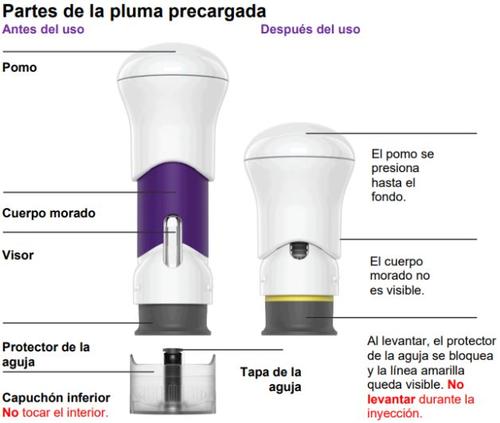
Stelara is presented in a single-dose pre-filled syringe that contains a dose of 45 mg or a dose of 90 mg.
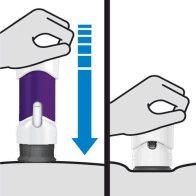
During injection, press the plunger until the purple part of the syringe is completely hidden. DO NOT LIFT THE PRE-FILLED SYRINGE during injection. If you do, the pre-filled syringe will lock and you will not receive the full dose. |
If your doctor considers that you or your caregiver are able to administer your Stelara injections at home, you will receive training to prepare and inject Stelara correctly using the pre-filled syringe. Do not attempt to administer the injections yourself without having received training from your doctor.
Each pre-filled syringe can only be used once. Dispose of the syringe (see step 3) after use, even if there is still medicine inside.
Do not reuse the pre-filled syringe.
Read these instructions for use before using the Stelara pre-filled syringe and each time you use a new pre-filled syringe.There may be new information. This leaflet does not replace the conversation with your doctor about your health or treatment.
If you cannot administer the injection yourself:
- ask your doctor or nurse for help, or
- ask someone who has been trained by a doctor or nurse to administer the injections.
To reduce the risk of accidental needlesticks, each pre-filled syringe has a needle shield that automatically covers the needle and locks after administering the injection and lifting the syringe. Do not lift the syringe until you have completed the injection.
The needle cap located inside the lower cap of the pre-filled syringe contains latex. Do not handle the needle cap if you are allergic to latex.
Also, read the leaflet carefully before starting to administer the injection and discuss any questions you have with your doctor or nurse.
 Storage Information
Storage Information
Store in a refrigerator between 2 °C and 8 °C. If necessary, store at room temperature up to 30 °C for a maximum of 30 days in the original carton. Do not refrigerate againonce stored at room temperature.
Do not freezethe pre-filled syringe.
Keep the pre-filled syringe and all medicines out of the reach of children.
Do not shakethe pre-filled syringe. Shaking can damage the Stelara medicine. If the pre-filled syringe has been shaken, do not use it. Use a new pre-filled syringe.
Store the pre-filled syringe in the original carton to protect it from light and physical damage.
 Need Help?
Need Help?
Consult your doctor with any questions you may have. For additional help or to share your experience, consult the contact information of your local representative in the leaflet.

- Preparation for Stelara Injection
Take the carton(s)
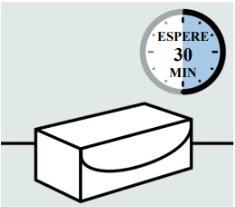
If it is refrigerated, remove the carton(s) of the pre-filled syringe from the refrigerator and place it on a flat surface.
Let it stand at room temperature for at least 30 minutesbefore use.
Do notheat it in any other way.
If your dose is 45 mg, you will receive a 45 mg pre-filled syringe. If your dose is 90 mg, you will receive a 90 mg pre-filled syringe or two 45 mg pre-filled syringes. If you receive two 45 mg pre-filled syringes, follow steps 1-3 for both injections. Choose a different injection site for the second injection |
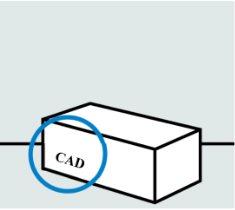
Check the expiration date ("EXPIRY DATE") and the carton seals
Do notuse the pre-filled syringe if the carton seals are broken or if it has passed the expiration date.
Do notuse the pre-filled syringe if it has been stored at room temperature for more than 30 days or if it has been stored above 30 °C. Consult your doctor or pharmacist to obtain a new pre-filled syringe.
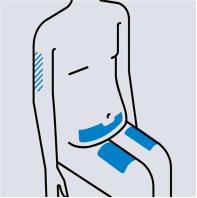
Choose the injection site
Choose from the following areas to administer the injection:
- Front of the thighs
- Lower abdomen (below the navel), avoiding the area 5 centimeters around the navel
If someone else is administering the injection, you can also use:
- The back of the arms
Do notinject into sensitive, bruised, red, or hardened skin.
Use a different injection site for each injection. |
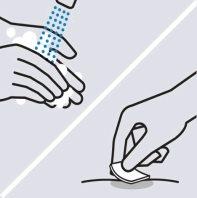
Wash your hands
Wash your hands well with warm water and soap.
Clean the injection site
Clean the chosen injection site with an alcohol wipe and let it dry.
Do nottouch, fan, or blow on the injection site after cleaning.
Inspect the liquid in the viewer
Choose a well-lit, clean, and flat work surface.
Remove the pre-filled syringe from the carton and check for damage.
Check the liquid in the viewer. It should be clear or slightly opalescentand colorless to light yellowand may contain small white or transparent particlesand one or more air bubbles. This is normal.
Do notinject if the liquid is frozen or cloudy, has an unusual color, or contains large particles.
Consult your doctor or pharmacist to obtain a new pre-filled syringe.
- Stelara Injection
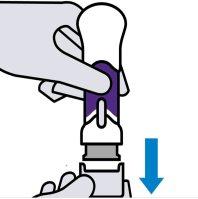
Remove the lower cap
Keep your hands away from the needle shieldafter removing the cap. It is normal to see a few drops of liquid.
Inject Stelara within 5 minutes of removing the cap.
Do notreplace the cap. This could damage the needle.
Do notuse a pre-filled syringe that has been dropped after removing the cap. Consult your doctor or pharmacist to obtain a new pre-filled syringe.
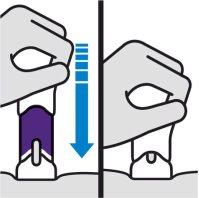
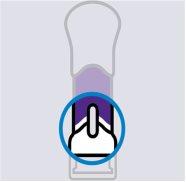
Apply directly to the skin. Press the plunger until it reaches the bottom, until the purple body is no longer visible.
DO NOT LIFT THE PRE-FILLED SYRINGE during injection. If you do, the needle shield will lock, showing a yellow line, and you will not receive the complete dose. |
You may hear a click when the injection starts. Continue pressing.
If you feel resistance, continue pressing. This is normal.
The medicine is injected as you press. Do it at a pace that feels comfortable for you.
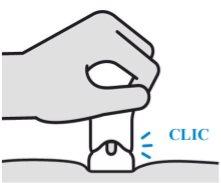
Confirm that your injection is complete
Your injection is complete when:
- The purple body is no longer visible.
- You cannot press the plunger further.
- You may hear a click.
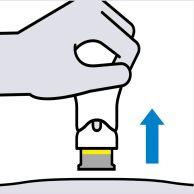
Lift the syringe straight up
The yellow line indicates that the needle shield has locked.
- After the Injection

Dispose of the pre-filled syringe
Place the used pre-filled syringe in a sharps container after use.
Do notthrow away (dispose of) the pre-filled syringes in household trash.
Do notrecycle the used sharps container.
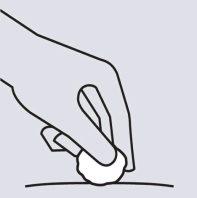
Inspect the injection site
There may be a small amount of blood or liquid at the injection site. This is normal.
Press the injection site with a cotton ball or gauze until it stops bleeding.
Do notrub the injection site.
If necessary, cover the injection site with a band-aid.
If you receive two 45 mg pre-filled syringes for a 90 mg dose, repeat steps 1-3 with the second pre-filled syringe. Choose a different injection site for the second injection. |
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to STELARA 90 mg SOLUTION FOR INJECTION IN PRE-FILLED PENDosage form: INJECTABLE PERFUSION, 130 mgActive substance: ustekinumabManufacturer: Accord Healthcare S.L.U.Prescription requiredDosage form: INJECTABLE, 45 mgActive substance: ustekinumabManufacturer: Accord Healthcare S.L.U.Prescription requiredDosage form: INJECTABLE, 90 mgActive substance: ustekinumabManufacturer: Accord Healthcare S.L.U.Prescription required
Online doctors for STELARA 90 mg SOLUTION FOR INJECTION IN PRE-FILLED PEN
Discuss questions about STELARA 90 mg SOLUTION FOR INJECTION IN PRE-FILLED PEN, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions









