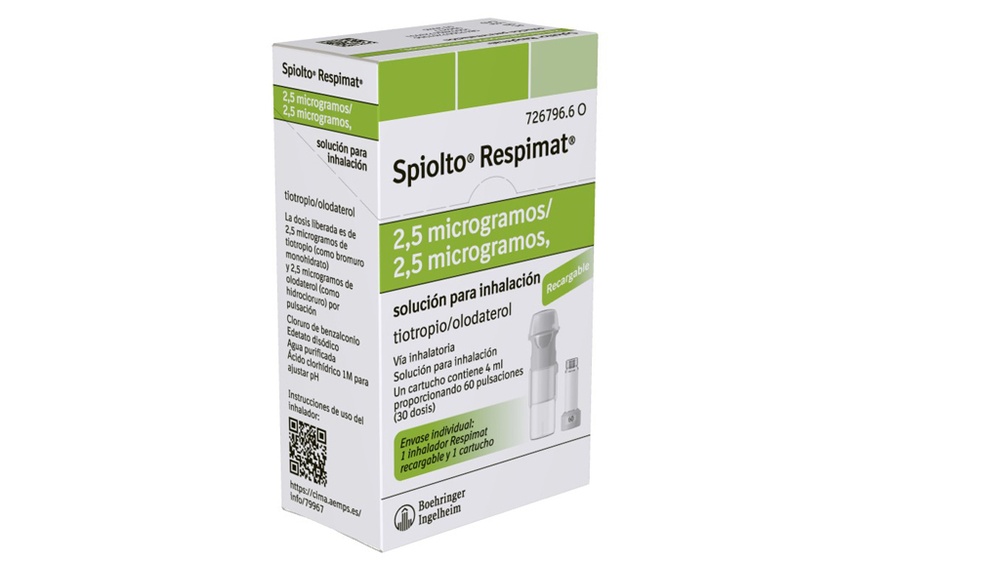
SPIOLTO RESPIMAT 2.5 micrograms/2.5 micrograms inhalation solution

How to use SPIOLTO RESPIMAT 2.5 micrograms/2.5 micrograms inhalation solution
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Spiolto Respimat 2.5 micrograms/2.5 micrograms, inhalation solution
tiotropium/olodaterol
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What Spiolto Respimat is and what it is used for
- What you need to know before you take Spiolto Respimat
- How to take Spiolto Respimat
- Possible side effects
- Storage of Spiolto Respimat
- Contents of the pack and other information
1. What Spiolto Respimat is and what it is used for
What Spiolto Respimat is
Spiolto Respimat contains two active substances called tiotropium and olodaterol. These belong to a group of medicines called long-acting bronchodilators. Tiotropium belongs to the subgroup of anticholinergics; olodaterol belongs to the subgroup of long-acting beta2 agonists.
What Spiolto Respimat is used for
Spiolto Respimat helps adult patients with chronic obstructive pulmonary disease (COPD) to breathe more easily. COPD is a long-term lung disease that makes it difficult to breathe and cough. The term COPD is associated with chronic bronchitis and emphysema.
Spiolto Respimat helps to open up the airways and makes it easier to breathe in and out. Regular use of Spiolto Respimat can also help when you have ongoing difficulty breathing due to your disease and will help minimize the effects of the disease on your daily life. Since COPD is a long-term disease, you should take Spiolto Respimat every day and not just when you have breathing problems or other COPD symptoms.
2. What you need to know before you take Spiolto Respimat
Do not take Spiolto Respimat
- if you are allergic to tiotropium or olodaterol or any of the other ingredients of this medicine (listed in section 6)
- if you are allergic to atropine or related substances, e.g. ipratropium or oxitropium.
Warnings and precautions
Consult your doctor or pharmacist before you take Spiolto Respimat
- if you have asthma (you should not take Spiolto Respimat for the treatment of asthma)
- if you have heart problems
- if you have high blood pressure
- if you have epilepsy
- if you have a specific thyroid gland problem called thyrotoxicosis
- if you have an abnormal enlargement of an artery called an aneurysm
- if you have diabetes
- if you have severe liver disease
- if you have kidney disease
- if you are scheduled for surgery
- if you have a problem in your eyes called narrow-angle glaucoma
- if you have prostate problems or difficulty urinating.
During treatment with Spiolto Respimat
- Stop taking the medicine and consult your doctor immediatelyif you feel a tightness in the chest, have a cough, wheezing or difficulty breathing immediately after taking the medication. These may be symptoms of a condition called bronchospasm (see section 4).
- If your breathing problems worsen or rashes, swelling, or itching occur immediately after using your inhaler, stop using it and consult your doctor immediately (see section 4).
- If you experience any side effects related to the heart (increased heart rate, increased blood pressure and/or increased symptoms such as chest pain), consult your doctor immediately (see section 4).
- If you experience muscle spasms, muscle weakness, or an abnormal heart rhythm, consult your doctor, as these symptoms may be related to low potassium levels in the blood (see section 4).
When using Spiolto Respimat, be careful not to get any spray in your eyes. This can cause eye pain or discomfort, blurred vision, halos around lights, or colored images associated with redness of the eyes (i.e., narrow-angle glaucoma). Eye symptoms may be accompanied by headache, nausea, or vomiting. Rinse your eyes with warm water, interrupt the intake of Spiolto Respimat, and consult your doctor immediately for more information.
Spiolto Respimat is indicated for the maintenance treatment of your chronic obstructive pulmonary disease. It should not be taken to treat a sudden episode of shortness of breath or wheezing.
Do not take Spiolto Respimat with certain medications that contain long-acting beta-adrenergic agonists, such as salmeterol or formoterol.
If you regularly take certain medications called short-acting beta-adrenergic agents, such as salbutamol, continue taking them only to relieve acute symptoms such as shortness of breath.
Dry mouth that has been observed with long-term anticholinergic treatment may be associated with tooth decay. Therefore, remember to take care of your oral hygiene.
Do not use Spiolto Respimat more than once a day.
Children and adolescents
Do notgive Spiolto Respimat to children or adolescents (under 18 years of age).
Other medicines and Spiolto Respimat
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines.
In particular, inform your doctor or pharmacist if you are taking:
- certain medications that may be similar to Spiolto Respimat (contain similar active substances such as anticholinergics or beta-adrenergic agents). You may be more likely to suffer from side effects.
- medications called beta-blockers used for high blood pressure or heart problems (such as propranolol) or for a problem in the eye known as glaucoma (such as timolol). This may reduce the effect of Spiolto Respimat.
- medications that decrease potassium levels in the blood. For example:
- corticosteroids (e.g., prednisolone),
- diuretics (medications to urinate),
- medications for respiratory problems such as theophylline.
Taking these medications with Spiolto Respimat may cause muscle spasms, muscle weakness, or an abnormal heart rhythm.
- medications called tricyclic antidepressants or MAO inhibitors (such as selegiline or moclobemide), which are used to treat neurological or psychiatric disorders such as Parkinson's disease or depression; the use of these medications will increase the risk of suffering from heart-related side effects.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before taking this medicine. You should not take this medicine unless your doctor has specifically recommended it.
Driving and using machines
No studies have been conducted on the effects on driving and using machines.
If you feel dizzy or experience blurred vision while taking Spiolto Respimat, do not drive or use tools or machines.
Spiolto Respimat contains Benzalkonium chloride
This medicine contains 0.0011 mg of benzalkonium chloride per puff.
Benzalkonium chloride may cause wheezing and breathing difficulties (bronchospasm), especially in patients with asthma.
3. How to take Spiolto Respimat
Follow the instructions for administration of this medicine exactly as indicated by your doctor. If in doubt, consult your doctor or pharmacist again.
Spiolto Respimat should only be used by inhalation.
Dosage
The recommended dose is:
Spiolto Respimat has a 24-hour effect, so you should take Spiolto Respimat only ONCE A DAY, if possible always at the same time of day. Each time you use it, inhale TWO PUFFS.
Since COPD is a long-term disease, you should take Spiolto Respimat every day and not just when you have difficulty breathing. Do not take more than the recommended dose.
Make sure you know how to use your Respimat rechargeable inhaler correctly. The instructions for use of the Respimat rechargeable inhaler are included at the end of this leaflet, see "Instructions on how to use the Respimat rechargeable inhaler".
Use in children and adolescents
There is no specific recommendation for the use of Spiolto Respimat in the pediatric population (under 18 years of age).
If you take more Spiolto Respimat than you should
You may have a greater risk of suffering from a side effect such as dry mouth, constipation, difficulty urinating, blurred vision, chest pain, high or low blood pressure, rapid or irregular heartbeat or palpitations, dizziness, nervousness, difficulty sleeping, anxiety, headache, tremors, muscle cramps, nausea, fatigue, discomfort, low potassium levels in the blood (which can cause muscle spasms, muscle weakness, or an abnormal heart rhythm), high blood sugar levels or too much acid in the blood (which can cause symptoms such as nausea, vomiting, weakness, muscle cramps, and rapid breathing).
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone 91 562 04 20, indicating the medicine and the amount ingested.
If you forget to take Spiolto Respimat
If you have forgotten to inhale a dose, inhale a single dose the next day at the usual time.
Do not use a double dose to make up for forgotten doses.
If you stop taking Spiolto Respimat
Before stopping treatment with Spiolto Respimat, you should talk to your doctor or pharmacist. If you stop treatment with Spiolto Respimat, the signs and symptoms of COPD may worsen.
If you have any further questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
If any of these reactions occur, please stop taking this medicineand consult your doctor immediately.
- Immediate allergic reactions to Spiolto Respimat are rare (may affect up to 1 in 1,000 people). These reactions may occur individually or as part of a severe allergic reaction (anaphylactic reaction) after administration of Spiolto Respimat. These include rash, hives, swelling of the mouth and face, sudden difficulty breathing (angioedema) or other hypersensitivity reactions (such as sudden drop in blood pressure or dizziness).
- As with all inhaled medications, some patients may experience chest tightness associated with coughing, wheezing, or difficulty breathing immediately after inhalation (paradoxical bronchospasm). The frequency cannot be estimated from the available data.
- Visual halos or colored images associated with redness of the eyes (glaucoma). The frequency cannot be estimated from the available data.
- Intestinal blockage or absence of bowel movement (intestinal obstruction including paralytic ileus). The frequency cannot be estimated from the available data.
Other possible side effects:
Uncommon (may affect up to 1 in 100 people)
- increased heart rate (tachycardia)
- dizziness
- headache
- cough
- hoarseness (dysphonia)
- dry mouth.
Rare (may affect up to 1 in 1,000 people)
- irregular heartbeat (atrial fibrillation)
- rapid heartbeat (supraventricular tachycardia)
- palpitations
- high blood pressure (hypertension)
- difficulty urinating (urinary retention)
- urinary tract infections
- painful urination (dysuria)
- throat inflammation (pharyngitis)
- larynx inflammation (laryngitis)
- gum inflammation (gingivitis)
- mouth inflammation (stomatitis)
- fungal infection in the mouth or throat (oropharyngeal candidiasis)
- nasal bleeding (epistaxis)
- difficulty sleeping (insomnia)
- blurred vision
- chest tightness associated with coughing, wheezing, or difficulty breathing immediately after inhalation (bronchospasm)
- constipation
- nausea
- itching (pruritus)
- joint pain (arthralgia)
- joint swelling
- back pain.
Not known (frequency cannot be estimated from the available data)
- increased eye pressure
- nasopharyngitis
- sinusitis
- difficulty swallowing (dysphagia)
- tongue inflammation (glossitis)
- heartburn (gastroesophageal reflux)
- tooth decay
- skin infections or ulcers
- dry skin
- dehydration.
You may experience side effects that occur with other medicines similar to Spiolto Respimat (beta-adrenergic agents) for treating respiratory problems. These may be irregular heartbeat, chest pain, low blood pressure, tremors, nervousness, muscle cramps, fatigue, discomfort, low potassium levels in the blood (which can cause muscle spasms, muscle weakness, or an abnormal heart rhythm), high blood sugar levels or too much acid in the blood (which can cause symptoms such as nausea, vomiting, weakness, muscle cramps, and rapid breathing).
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist, even if it is possible that they are not listed in this leaflet. You can also report side effects directly to the Spanish Medicines Agency's Pharmacovigilance System for Human Use: http://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Spiolto Respimat
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and on the label of the cartridge after EXP. The expiry date is the last day of the month stated.
Do not freeze.
Shelf life after first opening:
Replace the cartridge at the latest three months after insertion.
Do not use the Respimat rechargeable inhaler for more than one year.
Recommended use: 6 cartridges per inhaler.
Note: The functionality of the Respimat rechargeable inhaler has been demonstrated in tests for 540 puffs (corresponding to 9 cartridges).
Medicines should not be disposed of via wastewater or household waste. Return the cartridges and medicines you no longer need to the SIGRE collection point at your usual pharmacy. Ask your pharmacist how to dispose of cartridges and medicines you no longer need. This will help protect the environment.
6. Contents of the pack and additional information
Composition of Spiolto Respimat
The active ingredients are tiotropium and olodaterol. The delivered dose is 2.5 micrograms of tiotropium (as monohydrate bromide) and 2.5 micrograms of olodaterol (as hydrochloride) per actuation.
The delivered dose is the dose available to the patient after passing through the mouthpiece.
The other components are:
Benzalkonium chloride, disodium edetate, purified water, and hydrochloric acid to adjust the pH.
Appearance of the product and pack contents
Spiolto Respimat 2.5 micrograms/2.5 micrograms consists of a cartridge containing an inhalation solution and a Respimat inhaler. Before first use, insert the cartridge into the inhaler.
Single pack: 1 Respimat inhaler and 1 cartridge providing 60 actuations (30 doses).
Triple pack: 1 Respimat inhaler and 3 cartridges, each providing 60 actuations (30 doses).
Single replacement pack: 1 cartridge providing 60 actuations (30 doses).
Triple replacement pack: 3 cartridges, each providing 60 actuations (30 doses).
Not all pack sizes may be marketed.
Marketing authorisation holder and manufacturer
The marketing authorisation holder for Spiolto Respimat is:
Boehringer Ingelheim International GmbH
Binger Strasse 173
55216 Ingelheim am Rhein
Germany
The manufacturer of Spiolto Respimat is:
Boehringer Ingelheim Pharma GmbH & Co. KG
Binger Strasse 173
55216 Ingelheim am Rhein
Germany
Boehringer Ingelheim España, S.A.
Prat de la Riba, 50
08174 Sant Cugat del Vallès (Barcelona)
Spain
Boehringer Ingelheim France
100-104 Avenue de France
75013 Paris
France
Local representative:
Boehringer Ingelheim España, S.A.
Prat de la Riba, 50
08174 Sant Cugat del Vallès (Barcelona)
Spain
This medicinal product is authorised in the Member States of the European Economic Area and in the United Kingdom (Northern Ireland) under the following names:
Austria, Liechtenstein, Belgium, Luxembourg, Cyprus, Greece, Croatia, Czech Republic, Denmark, Estonia, France, Germany, Hungary, Iceland, Ireland, Malta, United Kingdom (Northern Ireland), Italy, Latvia, Lithuania, Netherlands, Norway, Poland, Portugal, Romania, Slovakia, Slovenia, Spain, Sweden: Spiolto Respimat
Finland: Inspiolto Respimat
Bulgaria: ??????? ????????
Date of last revision of this leaflet:December 2024
Detailed and up-to-date information on this medicinal product is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es.
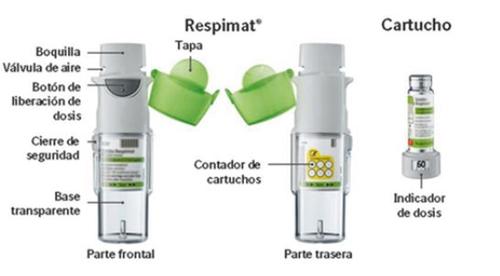
Instructions on how to use the Respimat rechargeable inhaler
Respimat is an inhaler device that generates an aerosol for inhalation. Respimat is for your use only. One cartridge provides several doses. The Respimat rechargeable inhaler allows you to replace the cartridge and can be used with up to 6 cartridges.
Read these instructions before starting to use Spiolto Respimat.
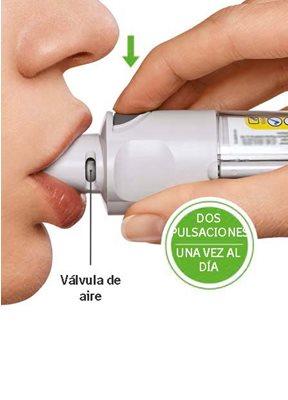
You will need to use this inhaler ONCE A DAY. Each time you use it, perform TWO ACTUATIONS.
- If Spiolto Respimat has not been used for more than 7 days, perform one actuation towards the ground.
- If Spiolto Respimat has not been used for more than 21 days, repeat the steps from 4 to 6 described in “Preparation for use” until you observe a cloud. Then repeat the steps from 4 to 6 three more times.
How to maintain your Respimat rechargeable inhaler
Clean the mouthpiece, including the metal part inside it, with a damp cloth or tissue, at least once a week.
Any slight discoloration of the mouthpiece does not affect the functioning of your Respimat rechargeable inhaler.
If necessary, clean the outside of your Respimat rechargeable inhaler with a damp cloth.
When to replace the inhaler
When you have used 6 cartridges with the same inhaler, get a new pack of Spiolto Respimat containing an inhaler. Do not use the Respimat rechargeable inhaler for more than one year after inserting the first cartridge.

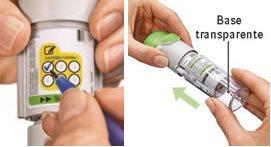
Preparation for use
|
|
|
|
|
|
|
|
|
|
Your inhaler is now ready to be used and will deliver 60 actuations (30 doses). |
|
Daily use
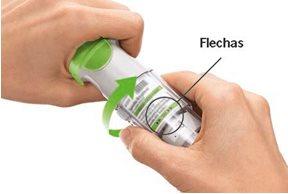
TURN
|
|
OPEN
|
|
ACTUATE
|
|
When to replace the Spiolto Respimat cartridge
The dose indicator shows how many actuations are left in the cartridge.
 60 actuations remaining.
60 actuations remaining.
 Less than 10 actuations remaining. Get a new cartridge.
Less than 10 actuations remaining. Get a new cartridge.
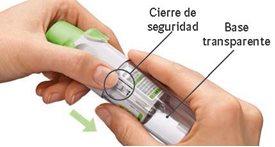
 Your cartridge is empty. Turn the transparent base to loosen it. Your inhaler is now locked. Remove the cartridge from the inhaler. Insert a new cartridge until it clicks (see step 2). The new cartridge will protrude more than the first cartridge (continue with step 3). Remember to replace the transparent base to unlock the inhaler.
Your cartridge is empty. Turn the transparent base to loosen it. Your inhaler is now locked. Remove the cartridge from the inhaler. Insert a new cartridge until it clicks (see step 2). The new cartridge will protrude more than the first cartridge (continue with step 3). Remember to replace the transparent base to unlock the inhaler.
Frequently asked questions
It is difficult to insert the cartridge deep enough.
Have you accidentally turned the transparent base before inserting the cartridge?Open the cap, press the dose release button, and then insert the cartridge.
Are you changing the cartridge?The new cartridge will protrude more than the first cartridge. Insert the cartridge until it clicks, then replace the transparent base.
I cannot actuate the dose release button.
Have you replaced the transparent base?If not, replace the transparent base to unlock the inhaler. The Respimat rechargeable inhaler only works with the transparent base in place.
Have you turned the transparent base?If not, turn the transparent base with a continuous movement until it clicks (half a turn). Does the dose indicator on your cartridge show a white arrow on a red background?Your cartridge is empty. Insert a new cartridge and replace the transparent base.
My Spiolto Respimat sprays automatically.
Was the cap open when you turned the transparent base?Close the cap, then turn the transparent base.
Did you press the dose release button while turning the transparent base?Close the cap, so that the dose release button is covered, then turn the transparent base.
Did you stop turning the transparent base before it clicked?Turn the transparent base with a continuous movement until it clicks (half a turn). The dose counter will count each incomplete turn and the number of remaining doses will decrease.
Was the cap open when you changed the cartridge?Close the cap, then change the cartridge.
Other sources of information
You can access detailed and up-to-date information on how to administer this medicinal product by scanning with your mobile phone (smartphone) the QR code included in the section “Instructions on how to use the Respimat rechargeable inhaler” of this leaflet and on the packaging. You can also access this information on the following internet address: https://cima.aemps.es/info/79967.

- Country of registration
- Average pharmacy price62.44 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to SPIOLTO RESPIMAT 2.5 micrograms/2.5 micrograms inhalation solutionDosage form: PULMONARY INHALATION, 2.5 micrograms/2.5 microgramsActive substance: olodaterol and tiotropium bromideManufacturer: Boehringer Ingelheim International GmbhPrescription requiredDosage form: PULMONARY INHALATION, 55 MICROGRAMS/22 MICROGRAMSActive substance: vilanterol and umeclidinium bromideManufacturer: Glaxosmithkline (Ireland) LimitedPrescription requiredDosage form: PULMONARY INHALATION, 340/12 microgramsActive substance: formoterol and aclidinium bromideManufacturer: Covis Pharma Europe B.V.Prescription required
Online doctors for SPIOLTO RESPIMAT 2.5 micrograms/2.5 micrograms inhalation solution
Discuss questions about SPIOLTO RESPIMAT 2.5 micrograms/2.5 micrograms inhalation solution, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions