
SPIKEVAX XBB.1.5 50 MICROGRAMS INJECTABLE DISPERSION

How to use SPIKEVAX XBB.1.5 50 MICROGRAMS INJECTABLE DISPERSION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Spikevax XBB.1.5 0.1 mg/ml injectable dispersion
Spikevax XBB.1.5 50 micrograms injectable dispersion
Spikevax XBB.1.5 50 micrograms injectable dispersion in pre-filled syringe
Spikevax XBB.1.5 25 micrograms injectable dispersion in pre-filled syringe
COVID-19 mRNA vaccine
andusomeran
This medicinal product is subject to additional monitoring, which will allow for the quick identification of new safety information. You can help by reporting any side effects you may get. The last section of the package leaflet contains information on how to report side effects.
Read all of this leaflet carefully before you receive this vaccine, because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist or nurse.
- If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Spikevax XBB.1.5 and what is it used for
- What you need to know before you receive Spikevax XBB.1.5
- How Spikevax XBB.1.5 is administered
- Possible side effects
- Storage of Spikevax XBB.1.5
- Contents of the pack and other information
1. What is Spikevax bivalent Original/Omicron BA.4-5 and what is it used for
Spikevax XBB.1.5 is a vaccine used to prevent COVID-19 caused by the SARS-CoV-2 virus. It is administered to adults and children from 6 years of age and older. The active substance of Spikevax XBB.1.5 is mRNA that encodes the spike protein of the SARS-CoV-2 virus. The mRNA is encapsulated in lipid nanoparticles SM-102.
Because Spikevax XBB.1.5 does not contain the virus, it cannot give you COVID-19.
How the vaccine works
Spikevax XBB.1.5 stimulates the body's natural defenses (immune system). The vaccine works by making the body produce protection (antibodies) against the virus that causes COVID-19. Spikevax XBB.1.5 uses a substance called messenger ribonucleic acid (mRNA) to carry instructions that the body's cells can use to produce the spike protein that is also found in the virus. Then, the cells make antibodies against the spike protein to help fight the virus. This will help protect you against COVID-19.
2. What you need to know before you receive Spikevax XBB.1.5
The vaccine must not be administered ifyou are allergic to the active substance or to any of the other components of this vaccine (listed in section 6).
Warnings and precautions
Talk to your doctor, pharmacist or nurse before you are given Spikevax XBB.1.5 if:
- you have previously had a severe, life-threatening allergic reactionafter any other vaccine or after receiving Spikevax (original) in the past.
- you have a very weak or damaged immune system.
- you have ever fainted after any injection with a needle.
- you have a bleeding disorder.
- you have a high fever or a severe infection; however, you can be vaccinated if you have a mild fever or an upper respiratory tract infection such as a cold.
- you have any serious illness.
- you have injection-related anxiety.
There is a higher risk of myocarditis (inflammation of the heart muscle) and pericarditis (inflammation of the outer lining of the heart) after vaccination with Spikevax (see section 4).
These disorders can occur a few days after vaccination and have mainly occurred within 14 days. They have been observed more frequently in young males and, more frequently, after the second dose of vaccination than after the first.
Most cases of myocarditis and pericarditis recover. Some of the cases required intensive care and fatal cases have been observed.
After vaccination, you should be alert to the signs of myocarditis and pericarditis, such as difficulty breathing, palpitations and chest pain, and should seek immediate medical attention if they occur.
If you are in any of the above situations (or are unsure), talk to your doctor, pharmacist or nurse before you are given Spikevax XBB.1.5.
Exacerbations of capillary leak syndrome
Some cases of exacerbation of capillary leak syndrome (which causes fluid to leak out of small blood vessels or capillaries, resulting in rapid swelling of the arms and legs, sudden weight gain and feeling of fainting and low blood pressure) have been reported after vaccination with Spikevax (original). If you have had previous episodes of capillary leak syndrome, talk to your doctor before you receive Spikevax XBB.1.5.
Duration of protection
As with any vaccine, the third dose of Spikevax XBB.1.5 may not fully protect all people who receive it and it is not known how long you will be protected.
Children
Spikevax XBB.1.5 is not recommended for use in children under 6 months.
Other medicines and Spikevax XBB.1.5
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines. Spikevax XBB.1.5 may affect the way other medicines work, and other medicines may affect the way Spikevax XBB.1.5 works.
People with weakened immune systems
The efficacy of Spikevax XBB.1.5 may be lower in people with weakened immune systems. In these cases, you should continue to maintain physical precautions to avoid COVID-19. Moreover, people close to you should be vaccinated as appropriate. Consult your doctor for individual recommendations.
Pregnancy and breastfeeding
If you are pregnant or think you may be pregnant, tell your doctor, nurse or pharmacist before you receive this vaccine. There is no data yet on the use of Spikevax XBB.1.5 during pregnancy. However, a large amount of observational data on pregnant women vaccinated with Spikevax (original) during the second and third trimester has not shown adverse effects on pregnancy or the newborn. Although data on pregnancy outcomes after vaccination during the first trimester are limited, no increased risk of spontaneous abortion has been observed. Since the differences between the two products are limited to the sequence of the spike protein in the vaccine and there are no clinically relevant differences, Spikevax XBB.1.5 can be used during pregnancy.
There is no data yet on the use of Spikevax XBB.1.5 during breastfeeding.
However, no effects on breastfed infants/children are expected. Data from women during breastfeeding after vaccination with Spikevax (original) have not shown a risk of adverse effects in breastfed infants/children. Spikevax XBB.1.5 can be administered during breastfeeding.
Driving and using machines
Do not drive or use machines if you feel unwell after vaccination. Wait until these effects have passed before driving or using machines.
Spikevax XBB.1.5 contains sodium
This medicinal product contains less than 1 mmol of sodium (23 mg) per dose; this is essentially “sodium-free”.
3. How Spikevax XBB.1.5 is administered
Table 1. Dosage of Spikevax XBB.1.5
Age(s) | Dose | Additional recommendations |
Children from 6 months to 4 years of age, without prior vaccination and without known history of SARS-CoV-2 infection. | Two doses of 0.25 ml each, administered intramuscularly* | Administer the second dose 28 days after the first dose. If a child has received a previous dose of Spikevax, a dose of Spikevax bivalent Original/Omicron BA.4-5 should be administered to complete the two-dose series. |
Children from 6 months to 4 years of age, with prior vaccination or known history of SARS-CoV-2 infection | One dose of 0.25 ml, administered intramuscularly* | Spikevax XBB.1.5 should be administered at least 3 months after the most recent dose of a COVID-19 vaccine. |
Children from 5 to 11 years of age, with or without prior vaccination | One dose of 0.25 ml, administered intramuscularly* | |
Persons 12 years of age and older, with or without prior vaccination | One dose of 0.5 ml, administered intramuscularly | |
Persons 65 years of age and older | One dose of 0.5 ml, administered intramuscularly | An additional dose may be administered at least 3 months after the most recent dose of a COVID-19 vaccine. |
- Do not use the single-dose vial or the pre-filled syringe to administer a partial volume of 0.25 ml.
Table 2. Dosage of Spikevax XBB.1.5 for immunocompromised individuals
Age(s) | Dose | Additional recommendations |
Immunocompromised children from 6 months to 4 years of age, without prior vaccination | Two doses of 0.25 ml administered intramuscularly* | In severely immunocompromised individuals, a third dose may be administered at least 28 days after the second dose. |
Immunocompromised children from 6 months to 4 years of age, with prior vaccination | One dose of 0.25 ml, administered intramuscularly* | In severely immunocompromised individuals, an additional dose (or doses) may be administered according to age, at least 2 months after the most recent dose of a COVID-19 vaccine, at the discretion of the healthcare professional, taking into account the individual's clinical circumstances. |
Immunocompromised children from 5 to 11 years of age, with or without prior vaccination | One dose of 0.25 ml, administered intramuscularly* | |
Immunocompromised individuals 12 years of age and older, with or without prior vaccination | One dose of 0.5 ml, administered intramuscularly |
- Do not use the single-dose vial or the pre-filled syringe to administer a partial volume of 0.25 ml.
Your doctor, pharmacist or nurse will inject the vaccine into a muscle (intramuscular injection) in the upper arm.
Aftereach injection of the vaccine, your doctor, pharmacist or nurse will observe you for at least 15 minutesto detect signs of an allergic reaction.
If you have any further questions on the use of this vaccine, ask your doctor, pharmacist or nurse.
4. Possible side effects
Like all medicines, this vaccine can cause side effects, although not everybody gets them.
Seek urgentmedical attention if you have any of the following signs and symptoms of an allergic reaction:
- dizziness or fainting;
- changes in heart rate;
- shortness of breath;
- wheezing;
- swelling of the lips, face or throat;
- rash or skin eruption;
- nausea or vomiting;
- stomach pain.
Talk to your doctor or nurse if you experience any other side effect. These may include:
Very common(may affect more than 1 in 10 people):
- swelling/pain on palpation in the armpit
- decreased appetite (observed in children from 6 months to 5 years)
- irritability/crying (observed in children from 6 months to 5 years)
- headache
- sleepiness (observed in children from 6 months to 5 years)
- nausea
- vomiting
- muscle and joint pain and stiffness
- pain or swelling at the injection site
- redness at the injection site (some of which may occur approximately between 9 and 11 days after injection)
- feeling very tired
- chills
- fever
Common(may affect up to 1 in 10 people):
- diarrhea
- skin rash
- skin rash or hives at the injection site (some of which may occur approximately between 9 and 11 days after injection)
Uncommon(may affect up to 1 in 100 people):
- itching at the injection site
- dizziness
- stomach pain
- elevated and itchy skin rash (urticaria) (which may appear from the time of injection to approximately two weeks after injection)
Rare(may affect up to 1 in 1,000 people):
- temporary one-sided facial drooping (Bell's palsy)
- facial swelling (facial swelling may occur in people who have received facial cosmetic injections)
- decreased sensation or pain on palpation of the skin
- unusual sensation in the skin, such as tingling (paresthesia)
Very rare(may affect up to 1 in 10,000 people):
- inflammation of the heart muscle (myocarditis) or inflammation of the outer lining of the heart (pericarditis) which may lead to difficulty breathing, palpitations or chest pain.
Frequency not known
- severe allergic reactions with difficulty breathing (anaphylaxis)
- allergic reactions with increased pain on palpation or intolerance of the immune system (hypersensitivity)
- a skin reaction that causes red spots or patches on the skin, which may look like a target or “bull's eye” with a dark red center surrounded by lighter red rings (erythema multiforme).
- extensive swelling of the vaccinated limb
- heavy menstrual bleeding (most cases were of moderate and temporary nature)
- rash caused by an external stimulus such as strong friction, scratching or pressure on the skin (mechanical urticaria)
- elevated and itchy skin rash lasting more than six weeks (chronic urticaria)
Reporting of side effects
If you experience side effects, talk to your doctor, pharmacist or nurse, even if they are not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this vaccine.
5. Storage of Spikevax XBB.1.5
Keep this vaccine out of the sight and reach of children.
Do not use this vaccine after the expiry date which is stated on the label after EXP. The expiry date is the last day of the month shown.
Information on storage, expiry and handling is described in the section for healthcare professionals at the end of the leaflet.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Contents and Additional Information
What Spikevax XBB.1-5 contains
Table 3. Composition of each container type
Concentration | Container | Dose | Composition per dose |
Spikevax XBB.1-5 0.1 ml injectable suspension injectable | Vial multidose of 2.5 ml | 5 doses of 0.5 ml each or a maximum of 10 doses of 0.25 ml each one | One dose (0.5 ml) contains 25 micrograms of elasomeran and 25 micrograms of davesomeran, an mRNA vaccine against COVID-19 (with modified nucleosides) (encapsulated in lipid nanoparticles SM-102). One dose (0.25 ml) contains 25 micrograms of andusomeran, an mRNA vaccine against COVID-19 (with modified nucleosides) (encapsulated in lipid nanoparticles SM-102). |
Spikevax XBB.1.5 50 μg injectable suspension | Vial single-dose of 0.5 ml | 1 dose of 0.5 ml For single use. | One dose (0.5 ml) contains 50 micrograms of andusomeran, an mRNA vaccine against COVID-19 (with modified nucleosides) (encapsulated in lipid nanoparticles SM-102). |
Spikevax XBB.1.5 50 μg injectable suspension in prefilled syringe | Prefilled syringe | 1 dose of 0.5 ml For single use | One dose (0.25 ml) contains 25 micrograms of andusomeran, an mRNA vaccine against COVID-19 (with modified nucleosides) (encapsulated in lipid nanoparticles SM-102). |
Andusomeran is a single-stranded messenger RNA (mRNA) with a 5' cap produced by in vitro acellular transcription from the corresponding DNA templates, which encodes the viral spike (S) protein of SARS-CoV-2 (omicron XBB.1.5).
The other components are lipid SM-102 (heptadecan-9-yl 8-{(2-hydroxyethyl)[6-oxo-6-(undecyloxy)hexyl]amino}octanoate), cholesterol, 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC), 1,2-dimyristoyl-rac-glycero-3-methoxypolyethylene glycol-2000 (PEG2000 DMG), trometamol, trometamol hydrochloride, acetic acid, sodium acetate trihydrate, sucrose, and water for injectable preparations.
Appearance of Spikevax XBB.1.5 and container contents
Spikevax XBB.1.5 0.1 mg/ml injectable suspension
Spikevax XBB.1.5 is a white to off-white suspension supplied in a 2.5 ml glass multidose vial with a rubber stopper and a removable plastic cap of blue color with an aluminum seal.
Container size: 10 multidose vials. Each vial contains 2.5 ml.
Spikevax XBB.1.5 50 micrograms injectable suspension
Spikevax XBB.1.5 is a white to off-white suspension supplied in a single-dose glass vial with a rubber stopper and a removable plastic cap of blue color with an aluminum seal.
Container sizes:
1 single-dose vial
10 single-dose vials
Each vial contains 0.5 ml.
Only some container sizes may be marketed.
Spikevax XBB.1.5 50 micrograms injectable suspension in prefilled syringe and Spikevax XBB.1.5 25 micrograms injectable suspension in prefilled syringe
Spikevax XBB.1.5 is a white to off-white suspension supplied in a prefilled syringe (cycloolefin copolymer) with a plunger stopper and a pointed cap (without needle).
The prefilled syringes are packaged in an inner paper tray within a cardboard box or in 1 transparent blister with 1 prefilled syringe or 5 transparent blisters with 2 prefilled syringes each.
Container sizes:
1 prefilled syringe
10 prefilled syringes
Only some container sizes may be marketed.
Marketing authorization holder
MODERNA BIOTECH SPAIN, S.L.
C/ Julián Camarillo nº 31
28037 Madrid
Spain
Manufacturers
Rovi Pharma Industrial Services, S.A.
Paseo de Europa, 50
- San Sebastián de los Reyes
Madrid
Spain
Moderna Biotech Spain, S.L.
C/ Julián Camarillo nº 31
28037 Madrid
Spain
Rovi Pharma Industrial Services, S.A.
Calle Julián Camarillo n.° 35
28037 Madrid,
Spain
Patheon Italia S.p.a.
Viale G.B. Stucchi, 110
20900 Monza
Italy
Patheon Italia S.p.A.
2 Trav. SX Via Morolense 5
03013 Ferentino (FR)
Italy
For further information on this medicinal product, please contact the local representative of the marketing authorization holder.
België/Belgique/Belgien Tél/Tel: 0800 81 460 | Lietuva Tel: 88 003 1114 |
| Luxembourg/Luxemburg Tél/Tel: 800 85 499 |
Ceská republika Tel: 800 050 719 | Magyarország Tel: 06 809 87488 |
Danmark Tlf.: 80 81 06 53 | Malta Tel: 8006 5066 |
Deutschland Tel: 0800 100 9632 | Nederland Tel: 0800 409 0001 |
Eesti Tel: 800 0044 702 | Norge Tlf: 800 31 401 |
Ελλáδα Τηλ: 008004 4149571 | Österreich Tel: 0800 909636 |
España Tel: 900 031 015 | Polska Tel: 800 702 406 |
France Tél: 0805 54 30 16 | Portugal Tel: 800 210 256 |
Hrvatska Tel: 08009614 | România Tel: 0800 400 625 |
Ireland Tel: 1800 800 354 | Slovenija Tel: 080 083082 |
Ísland Sími: 800 4382 | Slovenská republika Tel: 0800 191 647 |
Italia Tel: 800 928 007 | Suomi/Finland Puh/Tel: 0800 774198 |
Κúπρος Τηλ: 80091080 | Sverige Tel: 020 10 92 13 |
Latvija Tel: 80 005 898 |
Date of last revision of this leaflet: {DD month YYYY}.
Scan the code with a mobile device to obtain the leaflet in different languages.

Or visit the URL https://www.ModernaCovid19Global.com
Detailed information on this vaccine is available on the European Medicines Agency website: https://www.ema.europa.eu.
The leaflet can be found in all languages of the European Union/European Economic Area on the European Medicines Agency website.
This information is intended only for healthcare professionals:
Traceability
In order to improve the traceability of biological medicinal products, the name and batch number of the administered product must be clearly recorded.
Spikevax XBB.1.5 should be administered by a qualified healthcare professional.
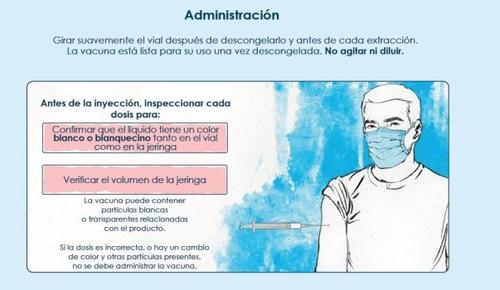
The vaccine is ready to use once thawed.
Do not shake or dilute.
The vaccine must be inspected visually for particles and color changes before administration.
Spikevax XBB.1.5 is a white to off-white suspension. It may contain white or translucent particles related to the product. Do not administer if the vaccine has changed color or contains other particles.
Frozen vaccine
The vials should be stored in a freezer between -50 °C and -15 °C.
Spikevax XBB.1.5 0.1 mg/ml injectable suspension (multidose vials with removable blue cap)
Five (5) doses (of 0.5 ml each) or a maximum of 10 doses (of 0.25 ml each) can be extracted from each multidose vial.
Puncture the stopper preferably at a different site each time.
Check that the vial has a removable blue cap and that the product name is Spikevax XBB.1.5. If the vial has a removable blue cap and the product name is Spikevax 0.1 mg/ml, Spikevax bivalent Original/Omicron BA.1 or Spikevax bivalent Original/Omicron BA.4-5, consult the summary of product characteristics for that formulation.
Thawed vaccine
The vaccine is shipped and supplied frozen or thawed. If the vaccine is frozen, thaw each multidose vial before use by following the instructions below (Table 4).
Table 4. Instructions for thawing multidose vials before use
Configuration | Instructions and thawing duration | |||
Thawing temperature (in refrigerator) | Thawing duration | Thawing temperature (at room temperature) | Thawing duration | |
Multidose vial | 2 °C-8 °C | 2 hours and 30 minutes | 15 °C-25 °C | 1 hour |
If the vaccine is received between 2 °C and 8 °C, it should be stored between 2 °C and 8 °C. The expiration date should have been updated on the outer packaging indicating the new expiration date between 2 °C and 8 °C.
During this period, it can be transported for 36 hours at a temperature between 2 °C and 8 °C.

Spikevax XBB.1.5 50 micrograms injectable suspension (single-dose vials)
The vaccine is ready to use once thawed.
Do not shake or dilute. Gently rotate the vial after thawing and before each extraction.
Check that the vial has a removable blue cap and that the product name is Spikevax XBB.1.5. If the vial has a removable blue cap and the product name is Spikevax bivalent Original/Omicron BA.1 or Spikevax bivalent Original/Omicron BA.4-5, consult the summary of product characteristics for that formulation.
Thawed vaccine
The vaccine is shipped and supplied frozen or thawed. If the vaccine is frozen, thaw each single-dose vial before use by following the instructions below. Each single-dose vial can be thawed separately or in the box of 1 or 10 units, either in the refrigerator or at room temperature (Table 5).
Table 5. Instructions for thawing single-dose vials and boxes before use
Configuration | Instructions and thawing duration | |||
Thawing temperature (in refrigerator) | Thawing duration | Thawing temperature (at room temperature) | Thawing duration | |
Single-dose vial | 2 °C-8 °C | 45 minutes | 15 °C-25 °C | 15 minutes |
Box | 2 °C-8 °C | 1 hour 45 minutes | 15 °C-25 °C | 45 minutes |
If the vaccine is received between 2 °C and 8 °C, it should be stored between 2 °C and 8 °C. The expiration date should have been updated on the outer packaging indicating the new expiration date between 2 °C and 8 °C.
During this period, it can be transported for 36 hours at a temperature between 2 °C and 8 °C.
Spikevax XBB.1.5 50 micrograms injectable suspension in prefilled syringe and Spikevax XBB.1.5 25 micrograms injectable suspension in prefilled syringe
Do not shake or dilute the contents of the prefilled syringe.
Each prefilled syringe is for single use. The vaccine is ready to use once thawed.
One (1) dose of 0.25 ml or 0.5 ml can be administered from each prefilled syringe, according to the volume specified on the syringe label. Do not use the 0.5 ml prefilled syringe to administer a 0.25 ml dose.
Spikevax XBB.1.5 is supplied in a single-dose prefilled syringe (without needle) containing 0.25 ml (25 micrograms of andusomeran) or 0.5 ml (50 micrograms of andusomeran) of mRNA and must be thawed before administration.
During storage, minimize exposure to ambient light and avoid exposure to direct sunlight and ultraviolet light.
Thawed vaccine
The vaccine is shipped and supplied frozen or thawed. If the vaccine is frozen, thaw each prefilled syringe before use by following the instructions below. The syringes can be thawed in the blisters (each blister contains 1 or 2 prefilled syringes, depending on the container size) or in the box itself, either in the refrigerator or at room temperature (Table 6).
Table 6. Instructions for thawing Spikevax XBB.1.5 prefilled syringes and boxes before use
Configuration | Instructions and thawing duration | |||
Thawing temperature (in refrigerator) (°C) | Thawing duration (minutes) | Thawing temperature (at room temperature) (°C) | Thawing duration (minutes) | |
Prefilled syringe In blister pack | 2-8 | 55 | 15-25 | 45 |
Box | 2-8 | 155 | 15-25 | 140 |
If the vaccine is received between 2 °C and 8 °C, it should be stored between 2 °C and 8 °C. The expiration date should have been updated on the outer packaging indicating the new expiration date between 2 °C and 8 °C.
The transportation duration of the prefilled syringe is limited by the duration for which the transport container is qualified.
Check that the product name on the prefilled syringe is Spikevax XBB.1.5. If the product name is Spikevax 50 micrograms, Spikevax bivalent Original/Omicron BA.1 or Spikevax bivalent Original/Omicron BA.4-5, consult the summary of product characteristics for that formulation.
Handling instructions for prefilled syringes
- Do not shake.
- Each prefilled syringe must be inspected visually for particles and color changes before administration.
- Spikevax bivalent Original/Omicron BA.1 is a white to off-white suspension. It may contain white or translucent particles related to the product. Do not administer if the vaccine has changed color or contains other particles.
- Needles are not included in the prefilled syringe boxes.
- Use a sterile needle of suitable size for intramuscular injection (21-gauge or finer needles).
- To remove the pointed cap, place it in a vertical position and turn it counterclockwise until it comes off. Remove the cap with a slow and continuous motion. Do not pull it while turning.
- Place the needle by turning it clockwise until the needle fits firmly into the syringe.
- Remove the needle cap when ready for administration.
- Administer the full dose by intramuscular route.
- Once thawed, do not refreeze.
Disposal
Disposal of unused medicinal product and all materials that have come into contact with it shall be done in accordance with local regulations.
Dosage and schedule
Table 7. Dosage of Spikevax XBB.1.5
Age(s) | Dose | Additional recommendations |
Children from 6 months to 4 years of age, without prior vaccination and without known history of SARS CoV-2 infection. | Two doses of 0.25 ml each administered intramuscularly* | Administer the second dose 28 days after the first If a child has received a previous dose of Spikevax, they should receive a dose of Spikevax XBB.1.5 to complete the series of two doses. |
Children from 6 months to 4 years of age, with prior vaccination or known SARS-CoV-2 infection | One dose of 0.25 ml, administered intramuscularly* | Spikevax XBB.1.5 should be administered at least 3 months after the most recent dose of a COVID-19 vaccine. |
Children from 5 to 11 years of age, with or without prior vaccination | One dose of 0.25 ml, administered intramuscularly* | |
Persons 12 years of age and older, with or without prior vaccination | One dose of 0.5 ml, administered intramuscularly | |
Persons 65 years of age and older | U |
In a dose of 0.5 ml, administered intramuscularly | A additional dose may be administered at least 3 months after the most recent dose of a COVID-19 vaccine. |
- Do not use the single-dose vial of 0.5 ml or the prefilled syringe of 0.5 ml to administer a partial volume of 0.25 ml.
Table 8. Dosage of Spikevax XBB.1.5 for Immunocompromised Individuals
Age(s) | Dose | Additional Recommendations |
Immunocompromised children from 6 months to 4 years of age, without prior vaccination | Two doses of 0.25 ml administered intramuscularly* | In severely immunocompromised individuals a third dose may be administered at least 28 days after the second dose. |
Immunocompromised children from 6 months to 4 years of age, with prior vaccination | One dose of 0.25 ml, administered intramuscularly* | In severely immunocompromised individuals one (or several) additional dose(s) may be administered according to age at least 2 months after the most recent dose of a COVID-19 vaccine, at the discretion of the healthcare professional, taking into account the individual's clinical circumstances. |
Immunocompromised children from 5 to 11 years of age, with or without prior vaccination | One dose of 0.25 ml, administered intramuscularly* | |
Immunocompromised individuals 12 years of age and older, with or without prior vaccination | One dose of 0.5 ml, administered intramuscularly |
- Do not use the single-dose vial of 0.5 ml or the prefilled syringe of 0.5 ml to administer a partial volume of 0.25 ml.
As with all injectable vaccines, adequate medical treatment and supervision should always be available in case of anaphylactic reaction after administration of Spikevax XBB.1.5.
Individuals will remain under observation by a healthcare professional for at least 15 minutes after vaccination.
There are no data to assess the concomitant administration of Spikevax XBB.1.5 with other vaccines. Spikevax XBB.1.5 should not be mixed with other vaccines or medications in the same syringe.
Administration
The vaccine should be administered intramuscularly. The most suitable site is the deltoid muscle of the arm. Do not administer this vaccine intravascularly, subcutaneously, or intradermally.
Multidose vials
Prefilled syringes
Use a sterile needle of the appropriate size for intramuscular injection (needles of 21 gauge or finer). To remove the needle shield, place it in a vertical position and twist it counterclockwise until it comes off. Remove the shield with a slow and continuous motion. Do not pull it while twisting. Place the needle by twisting it clockwise until it fits firmly onto the syringe. Remove the needle shield when ready for administration. Administer the complete dose intramuscularly. Discard the syringe after use. For single use only.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to SPIKEVAX XBB.1.5 50 MICROGRAMS INJECTABLE DISPERSIONDosage form: INJECTABLE, 0.1 mg/mlActive substance: covid-19, RNA-based vaccineManufacturer: Biontech Manufacturing GmbhPrescription requiredDosage form: INJECTABLE, 0.2 mLActive substance: covid-19, RNA-based vaccineManufacturer: Biontech Manufacturing GmbhPrescription requiredDosage form: INJECTABLE, 0.3 micrograms/0.3 mlActive substance: covid-19, RNA-based vaccineManufacturer: Biontech Manufacturing GmbhPrescription required
Online doctors for SPIKEVAX XBB.1.5 50 MICROGRAMS INJECTABLE DISPERSION
Discuss questions about SPIKEVAX XBB.1.5 50 MICROGRAMS INJECTABLE DISPERSION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions
















