SPEXOTRAS 0.05 mg/mL ORAL SOLUTION POWDER

How to use SPEXOTRAS 0.05 mg/mL ORAL SOLUTION POWDER
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Spexotras 0.05mg/ml powder for oral solution
trametinib
Read all of this leaflet carefully before your child starts taking this medicine, because it contains important information.
- Keep this leaflet, as you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for your child only. Do not give it to others, as it may harm them, even if they have the same symptoms as your child.
- If your child experiences side effects, consult your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. See section 4.
- The information in this leaflet is for you or your child, but the leaflet will only say "your child".
Contents of the Package Leaflet
- What is Spexotras and what is it used for
- What you need to know before giving Spexotras
- How to give Spexotras
- Possible side effects
- Storage of Spexotras
- Contents of the pack and further information
1. What is Spexotras and what is it used for
Spexotras is a medicine that contains the active substance trametinib.
It is used in combination with another medicine (dabrafenib dispersible tablets) in children from 1 year of age to treat a type of brain tumor called glioma.
Spexotras may be used in patients with:
- low-grade glioma
- high-grade glioma when the patient has received at least one treatment with radiation and/or chemotherapy.
Spexotras, in combination with dabrafenib dispersible tablets, is used to treat patients whose brain tumor has a specific mutation (change) in a gene called BRAF. This mutation causes the body to produce defective proteins that, in turn, can cause the development of the tumor. The doctor will assess this mutation before starting treatment.
In combination with dabrafenib, Spexotras targets these defective proteins and slows down or stops the development of the tumor. Also, read the package leaflet of dabrafenib dispersible tablets.
2. What you need to know before giving Spexotras
Do not give Spexotras
- if your child is allergicto trametinib or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Consult your doctor before starting to give Spexotras. The doctor needs to know if your child:
- has heart problemssuch as heart failure or problems with the way the heart beats.
- has or has ever had lung or breathing problems, such as difficulty breathing often accompanied by dry cough, shortness of breath, and fatigue.
- has eye problems, such as blockage of the veins that supply the eye (retinal vein occlusion) or swelling of the eye that may be due to fluid loss (chorioretinopathy).
- has or has had liver problems.
- has or has ever had kidney problems
- has or has had gastrointestinal problemssuch as diverticulitis (inflamed bags in the colon) or metastases in the gastrointestinal tract.
Before your child starts taking Spexotras, during, and after treatment, the doctor will perform checks to avoid complications.
Skin examination
Treatment may cause skin cancer. In general, these skin changes remain local and can be removed by surgery, and treatment can continue without interruption. The doctor may review your child's skin before and regularly during treatment.
Check your child's skin monthly during treatment and for 6 months after stopping this medicine and report to the doctoras soon as possible if you notice any changes in your child's skin such as a new wart, skin sore, or a reddish lump that bleeds or does not heal, or a change in the size or color of a mole.
Tumor lysis syndrome
If your child experiences the following symptoms, report to the doctor immediately, as it may be a potentially life-threatening condition: nausea, difficulty breathing, irregular heartbeats, muscle cramps, seizures, cloudy urine, decreased urine production, and fatigue. These may be caused by a group of metabolic complications that can occur during cancer treatment and are caused by the breakdown products of dying cancer cells (tumor lysis syndrome or TLS) and can cause changes in kidney function (see also section 4).
Childrenunder 1year of age
Spexotras in combination with dabrafenib dispersible tablets has not been evaluated in children under 1 year of age. Therefore, Spexotras is not recommended in this age group.
Patient over 18years of age
Information on treatment in patients over 18 years of age with glioma is limited, so the doctor must assess the continuation of treatment until adulthood.
Other medicines andSpexotras
Before starting treatment, tell your doctor, pharmacist, or nurse if your child is taking, has recently taken, or might take any other medicines, including medicines used to thin the blood or any other medicine obtained without a prescription.
Pregnancy,breast-feeding, and fertility
Pregnancy
- If your daughter is pregnant or thinks she may be pregnant, consult your doctor or nurse before using this medicine. Spexotras may harm the fetus.
- If your daughter becomes pregnant while taking this medicine, consult your doctor immediately.
Breast-feeding
It is not known if Spexotras can pass into breast milk. If your daughter is breast-feeding or plans to breast-feed, she must tell her doctor. You, your daughter, and the doctor will decide whether to take Spexotras or breast-feed.
Fertility
Spexotras may affect fertility in both males and females.
Taking Spexotras with dabrafenib dispersible tablets: Dabrafenib may reduce sperm count and may not return to normal levels until after stopping treatment with dabrafenib.
Before starting treatment with dabrafenib dispersible tablets, talk to your doctor about options to improve the chances of your child having children in the future.
Contraception
- If your daughter can become pregnant, she must use a reliable method of birth control (contraceptive) while taking Spexotras and for at least 16 weeks after stopping it.
- Hormonal birth control methods (such as the pill, injections, or patches) may not be as effective when taking Spexotras in combination with dabrafenib dispersible tablets. Therefore, while taking this combination of medicines, your daughter needs to use another effective birth control method to avoid becoming pregnant. Consult your doctor or nurse.
Driving and using machines
Spexotras may cause side effects that can affect your child's ability to drive, ride a bike/motorcycle, use machines, or participate in other activities that require being alert. If your child has vision problems or feels dizzy or weak, or feels a lack of energy, they should avoid such activities.
The description of side effects can be found in section 4. For more information, read the entire package leaflet.
If you are unsure, talk to your doctor, pharmacist, or nurse. Your child's ability to perform these activities may also be affected by their own illness, symptoms, or treatment.
Spexotras contains a cyclodextrin
This medicine contains 100 mg of a cyclodextrin in each ml of Spexotras oral solution.
Spexotras contains methyl parahydroxybenzoate
It may cause allergic reactions (possibly delayed).
Spexotras contains sodium
This medicine contains 1.98 mg of sodium (main component of table/cooking salt) in each ml of Spexotras oral solution. This is equivalent to 4% of the maximum recommended daily intake of sodium for an adult at the maximum daily dose of trametinib.
Spexotras contains potassium
This medicine contains potassium, less than 1 mmol (39 mg) per maximum daily dose; this is, essentially "potassium-free".
3. How to give Spexotras
Follow exactly the administration instructions of this medicine to your child as indicated by the doctor, pharmacist, or nurse. In case of doubt, consult the doctor, pharmacist, or nurse again.
How much to give
The doctor will decide the correct dose of Spexotras based on your child's body weight.
The doctor will decide if your child needs to take a lower dose according to the side effects they have.
How to give it
Read the instructions for use at the end of this leaflet for more information on how to give the oral solution. Your pharmacist will prepare the oral solution for you.
- Give Spexotras once a day. Giving Spexotras at the same time every day will help you remember when to give the medicine. Give Spexotras eitherwith the morning orevening dose of dabrafenib dispersible tablets. The doses of dabrafenib should be given with a difference of about 12 hours.
- Give Spexotras on an empty stomach, at least 1 hour before or 2 hours after a meal, which means:
- after taking Spexotras, your child must wait at least 1hourbefore eating.
- after eating, your child must wait at least 2hoursbefore taking Spexotras.
- if necessary, breast milk and/or formula can be given on demand.
If you give moreSpexotrasthan you should
If you give too much Spexotras, contact your doctor, pharmacist, or nurse immediately. If possible, show them the Spexotras packaging with the package leaflet.
If you forget to give Spexotras
If it has been less than 12 hours since the missed dose, give it as soon as you remember.
If it has been 12 hours or more since the missed dose, do not make up for the missed dose. Give the next dose at the usual time and then continue giving Spexotras at the usual time.
Do not give a double dose to make up for missed doses.
If your child vomits after taking Spexotras
If your child vomits after taking Spexotras, do not give another dose until the next scheduled dose.
If you interrupt treatment with Spexotras
Give Spexotras for the time your doctor has indicated. Do not interrupt treatment unless your doctor tells you to.
If you have any other questions about the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Stop giving this medicine and seek urgent medical attention if your child has any of the following symptoms:
- coughing up blood, blood in the urine, vomiting blood, or if the vomit looks like "coffee grounds", or if they have black, tarry stools. These may be signs of bleeding.
- fever (temperature of 38°C or higher).
- chest pain or difficulty breathing, sometimes with fever or cough. These may be signs of pneumonitis or inflamed lungs (interstitial lung disease).
- blurred vision, loss of vision, or other changes in vision. These may be signs of retinal detachment.
- redness of the eyes, eye pain, increased sensitivity to light. These may be signs of uveitis.
- unexplained muscle pain, muscle cramps, or muscle weakness, dark urine. These may be signs of rhabdomyolysis.
- severe abdominal pain. This may be a sign of pancreatitis.
- fever, swollen lymph nodes, bruising, or a rash at the same time. These may be signs of a condition in which the immune system produces too many cells that fight infections, which can cause various symptoms (hemophagocytic lymphohistiocytosis).
- nausea, difficulty breathing, irregular heartbeats, muscle cramps, seizures, cloudy urine, decreased urine production, and fatigue. These may be signs of a condition resulting from the rapid breakdown of cancer cells that in some people can be life-threatening (tumor lysis syndrome or TLS).
- red patches on the trunk, circular or target-like, with or without central blisters, skin peeling, ulcers in the mouth, throat, nose, genitals, and eyes. These may be signs of severe skin reactions that can be life-threatening and may be preceded by fever and flu-like symptoms (Stevens-Johnson syndrome), widespread rash, fever, and swollen lymph nodes (DRESS).
Other possible side effects
Very common(may affect more than 1 in 10 people)
- headache
- dizziness
- cough
- diarrhea, feeling sick (nausea), being sick (vomiting), constipation, stomach pain
- skin problems such as rash, acne-like rash, dry skin or itchy skin, redness of the skin
- warty growths (cutaneous papilloma)
- infection of the skin under the nails
- pain in arms or legs or joints
- lack of energy or feeling weak or tired
- weight gain
- infections of the upper respiratory tract with symptoms such as sore throat and nasal congestion (nasopharyngitis)
- increased liver enzymes in blood tests
- low levels of white blood cells (neutropenia, leucopenia)
- low levels of red blood cells (anemia)
Common(may affect up to 1 in 10 people)
- frequent urination with pain or burning (urinary tract infection)
- skin effects including skin infection (cellulitis), inflammation of the hair follicles in the skin, inflamed and peeling skin (generalized exfoliative dermatitis), thickening of the outer layer of the skin (hyperkeratosis)
- decreased appetite
- low blood pressure (hypotension)
- high blood pressure (hypertension)
- shortness of breath
- mouth ulcers or mouth pain, inflammation of the mucous membranes
- inflammation of the fatty tissue under the skin (panniculitis)
- unusual hair loss or thinning hair
- redness, pain in hands and feet (hand-foot syndrome)
- muscle spasms
- chills
- allergic reaction (hypersensitivity)
- dehydration
- vision problems including blurred vision
- slow heart rate (bradycardia)
- fatigue, chest discomfort, feeling dizzy, palpitations (reduced ejection fraction)
- localized swelling of the tissues (edema)
- muscle pain (myalgia)
- fatigue, chills, sore throat, joint or muscle pain (flu-like illness)
- abnormal test results related to creatine phosphokinase, an enzyme found mainly in the heart, brain, and skeletal muscle
- increased blood sugar levels
- low sodium or phosphate levels in the blood
- decreased platelet count in the blood (cells that help blood clot)
- increased sensitivity of the skin to the sun
Uncommon(may affect up to 1 in 100 people)
- irregular heartbeats (atrioventricular block)
- inflammation of the intestines (colitis)
- cracked skin
- night sweats
- excessive sweating
- purple-colored skin patches, painful, itchy, or blistering, mainly on the arms, legs, face, and neck, with fever (signs of acute febrile neutrophilic dermatosis)
In addition to the side effects described above, the following side effects have only been reported so far in adult patients, but may also occur in children:
- nervous system problems that can cause pain, loss of sensation, or tingling in the hands and feet and/or muscle weakness (peripheral neuropathy)
- dry mouth
- kidney failure
- benign skin tumor (acrocordon)
- inflammatory disease that mainly affects the skin, lungs, eyes, and lymph nodes (sarcoidosis)
- kidney inflammation
- hole (perforation) in the stomach or intestines
- inflammation of the heart muscle that can cause difficulty breathing, fever, palpitations, and chest pain
Reporting of side effects
If your child experiences any side effects, talk to your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. You can also report them directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Spexotras
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label of the vial and on the carton, after EXP. The expiry date refers to the last day of the month stated.
Store in the original packaging to protect from light and moisture.
Before reconstitution: Store in a refrigerator (between 2 °C and 8 °C).
After reconstitution: Store below 25 °C. Do not freeze. Discard any unused solution after 35 days following reconstitution.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and further information
Composition ofSpexotras
- The active substance is trametinib. One vial contains trametinib dimethyl sulfoxide equivalent to 4.7 mg of trametinib. Each ml of reconstituted solution contains 0.05 mg of trametinib.
- The other ingredients are: sulfobutyl ether beta-cyclodextrin sodium (see section 2), sucralose (E 955), citric acid monohydrate (E 330), disodium phosphate (E 339) (see section 2), potassium sorbate (E 202) (see section 2), methyl parahydroxybenzoate (E 218) (see section 2), and strawberry flavor.
Appearance and packaging of the product
Spexotras 0.05 mg/ml powder for oral solution is a white or almost white powder.
Spexotras is supplied in a 180 ml amber glass vial with a child-resistant screw cap, containing 12 g of powder. Each carton contains one vial, a pressure adapter for the vial, and a 20 ml reusable oral syringe with graduated markings of 0.5 ml.
Marketing authorisation holder
Novartis Europharm Limited
Vista Building
Elm Park, Merrion Road
Dublin 4
Ireland
Manufacturer
Sandoz S.R.L.
Str. Livenzeni nr.7A
540472 Targu Mures
Romania
Novartis Pharma GmbH
Roonstrasse 25
90429 Nuremberg
Germany
Novartis Farmacéutica S.A.
Gran Via de les Corts Catalanes 764
08013 Barcelona
Spain
Novartis Pharma GmbH
Sophie-Germain-Strasse 10
90443 Nürnberg
Germany
You can request more information about this medicine from the local representative of the marketing authorisation holder:
België/Belgique/Belgien Novartis Pharma N.V. Tél/Tel: +32 2 246 16 11 | Lietuva SIA Novartis Baltics Lietuvos filialas Tel: +370 5 269 16 50 |
| Luxembourg/Luxemburg Novartis Pharma N.V. Tél/Tel: +32 2 246 16 11 |
Ceská republika Novartis s.r.o. Tel: +420 225 775 111 | Magyarország Novartis Hungária Kft. Tel.: +36 1 457 65 00 |
Danmark Novartis Healthcare A/S Tlf.: +45 39 16 84 00 | Malta Novartis Pharma Services Inc. Tel: +356 2122 2872 |
Deutschland Novartis Pharma GmbH Tel: +49 911 273 0 | Nederland Novartis Pharma B.V. Tel: +31 88 04 52 111 |
Eesti SIA Novartis Baltics Eesti filiaal Tel: +372 66 30 810 | Norge Novartis Norge AS Tlf: +47 23 05 20 00 |
Ελλάδα Novartis (Hellas) A.E.B.E. Τηλ: +30 210 281 17 12 | Österreich Novartis Pharma GmbH Tel: +43 1 86 6570 |
España Novartis Farmacéutica, S.A. Tel: +34 93 306 42 00 | Polska Novartis Poland Sp. z o.o. Tel.: +48 22 375 4888 |
France Novartis Pharma S.A.S. Tél: +33 1 55 47 66 00 | Portugal Novartis Farma - Produtos Farmacêuticos, S.A. Tel: +351 21 000 8600 |
Hrvatska Novartis Hrvatska d.o.o. Tel. +385 1 6274 220 | România Novartis Pharma Services Romania SRL Tel: +40 21 31299 01 |
Ireland Novartis Ireland Limited Tel: +353 1 260 12 55 | Slovenija Novartis Pharma Services Inc. Tel: +386 1 300 75 50 |
Ísland Vistor hf. Sími: +354 535 7000 | Slovenská republika Novartis Slovakia s.r.o. Tel: +421 2 5542 5439 |
Italia Novartis Farma S.p.A. Tel: +39 02 96 54 1 | Suomi/Finland Novartis Finland Oy Puh/Tel: +358 (0)10 6133 200 |
Κύπρος Novartis Pharma Services Inc. Τηλ: +357 22 690 690 | Sverige Novartis Sverige AB Tel: +46 8 732 32 00 |
Latvija SIA Novartis Baltics Tel: +371 67 887 070 |
Date of last revision of this prospectus:
Other sources of information
Detailed information on this medicine is available on the European Medicines Agency website: https://www.ema.europa.eu.
This information is intended only for the pharmacist:
Reconstitution instructions (for pharmacist only):
- Wash and dry your hands.
- Check the expiry date of the powder in the vial.
- Gently tap the vial to loosen the powder.
- Remove the cap and add 90 ml of distilled or purified water to the powder in the vial.
- Replace the cap and invert the vial repeatedly for up to 5 minutes until completely dissolved. You can also shake gently.
- Separate the adapter from the vial and the oral syringe. Remove the cap from the vial and insert the adapter into the neck of the vial. Press firmly until the adapter is fully inserted. The adapter should be fully flush with the neck of the vial.
- Record the preparation date on the carton. The solution expires 35 days after preparation.
- Inform the recipient of the dose and the date the solution was prepared.
INSTRUCTIONS FOR USE
Ask your healthcare professional or pharmacist to show you how to use Spexotras correctly. Follow exactly the administration instructions of Spexotras indicated by your healthcare professional or pharmacist.
If you have any doubts about how to use Spexotras, consult your healthcare professional or pharmacist.
SECTIONAADMINISTRATION WITH THE ORAL SYRINGE | |
Vial adapter (already inserted into the vial neck) Solution in the vial Oral syringe In case of spillage or contact of the Spexotras solution with skin or eyes, follow the information in the "SPILL CLEANUP" section. Wash and dry your hands before administering Spexotras.
| |
1 Check the preparation date of the solution on the carton. Do notadminister Spexotras if more than 35 days have passed since the solution was prepared. Note:The expiry date printed on the right side of the vial label does notcorrespond to that of the solution. This expiry date applies only to the powder before it is reconstituted into a solution by the pharmacist. | |
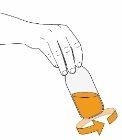
2 Gently shake the vial in circles for 30 seconds to mix the solution. If foam appears, let the vial stand until the foam disappears. |
|
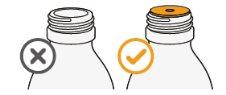
3 Remove the child-resistant cap by pressing down and twisting in a counter-clockwise direction. | |
4 Check if the vial adapter is already inserted into the vial neck. If it is not inserted, contact your pharmacist. |
|
5 Push the plunger down on the oral syringe to the end to eliminate all air from the inside. |
|
6 Place the vial on a flat surface and keep it in a vertical position. Insert the tip of the oral syringe into the adapter opening. Make sure the oral syringe is well connected. IMPORTANT:Due to air pressure, the plunger may move on its own when measuring your dose during Step 7. Hold the plunger to prevent it from moving. |
|
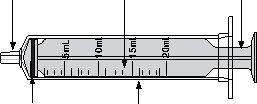
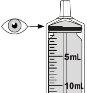
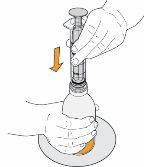
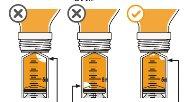
7 Carefully turn the vial upside down and pull the plunger to measure your dose. With the tip facing up, the topof the black cap should align with the prescribed dose. If large air bubbles appear in the syringe, as shown in the images, return the medication to the vial and withdraw your dose again. Continue doing this until there are no large air bubbles. Small air bubbles are acceptable. |
|
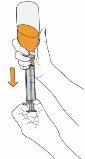
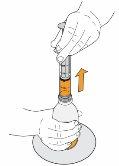
8 Continue holding the plunger in place, turn the vial back over, and place it on a flat surface. Pull the oral syringe out of the vial by gently pulling upwards. |
|
9 Re-check that the topof the black cap is at the prescribed dose. If it is not, repeat Steps 6 to 8. If administration is via the oral syringe, proceed to Step 10. If administration is through a feeding tube, go to "SECTION B". |
|
10 Place the tip of the oral syringe inside the mouth with the tip touching the inside of either cheek. Slowly press the plunger down to the bottom to administer the full dose. WARNING:Administering Spexotras directly into the throat or pushing the plunger too quickly can cause choking. |
|
11 Check that no Spexotras remains in the oral syringe. If any solution remains in the oral syringe, administer it. Note:If your dose is greater than the capacity of the oral syringe, repeat administration until the total volume is administered. | |
12 Replace the cap on the vial and turn it clockwise to close. Make sure the cap is securely tightened on the vial. Do not removethe vial adapter. | |
13 Clean the oral syringe following the instructions in "SECTION C", then store the solution and the oral syringe according to the instructions in the "STORAGE" section. |
SECTIONBADMINISTRATION THROUGH A FEEDING TUBE |
Please follow this section onlyif you are going to administer Spexotras through a feeding tube. To administer through a feeding tube, read the following information and proceed to Step 1.
|
1 Clean the feeding tube according to the manufacturer's instructions immediately before administering Spexotras. |
2 Follow Steps 1 to 9 of "SECTION A", then continue with Step 3 of this section. |
3 Connect the 20 ml oral syringe containing Spexotras to the feeding tube. You may need an ENFIT adapter to connect the oral syringe to the feeding tube. |
4 Apply constant pressure to dispense the solution into the feeding tube. |
5 Check that no Spexotras remains in the oral syringe. If any solution remains in the oral syringe, administer it. |
6 Re-clean the feeding tube according to the manufacturer's instructions. |
7 Go to "SECTION C" for cleaning. |
SECTIONCCLEANING |
To avoid contact of Spexotras with other kitchen utensils, always clean the oral syringe separately from other kitchen utensils. Cleaning of the oral syringe:
|
SPILL CLEANUP |
If Spexotras comes into contact with your skin, wash the area well with water and soap. If Spexotras comes into your eyes, rinse them with water. Follow these steps if some Spexotras solution spills:
|
STORAGE |
Keep the Spexotras solution and the oral syringe out of the sight and reach of children. Store the solution in a vertical position, in the provided carton with the cap perfectly closed. Store below 25 °C. Do notfreeze. Store the oral syringe in the provided carton along with the Spexotras solution. |
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to SPEXOTRAS 0.05 mg/mL ORAL SOLUTION POWDERDosage form: TABLET, 0.5 mgActive substance: trametinibManufacturer: Novartis Europharm LimitedPrescription requiredDosage form: TABLET, 2 mgActive substance: trametinibManufacturer: Novartis Europharm LimitedPrescription requiredDosage form: TABLET, 20 mgActive substance: cobimetinibManufacturer: Roche Registration GmbhPrescription required
Online doctors for SPEXOTRAS 0.05 mg/mL ORAL SOLUTION POWDER
Discuss questions about SPEXOTRAS 0.05 mg/mL ORAL SOLUTION POWDER, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions






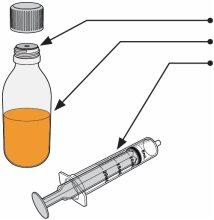 To administer Spexotras, you will need:
To administer Spexotras, you will need: