
SOOLANTRA 10 mg/g CREAM

How to use SOOLANTRA 10 mg/g CREAM
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Soolantra 10 mg/g Cream
Ivermectin
Read the entire package leaflet carefully before starting to take this medication, as it contains important information for you.
- Keep this package leaflet, as you may need to read it again. If you have any questions, ask your doctor or pharmacist.
- This medication has been prescribed to you only, and you should not give it to others, even if they have the same symptoms as you, as it may harm them.
- If you experience side effects, consult your doctor or pharmacist, even if they are not listed in this package leaflet. See section 4.
Contents of the Package Leaflet
- What is Soolantra and what is it used for
- What you need to know before using Soolantra
- How to use Soolantra
- Possible side effects
- Storage of Soolantra
- Package Contents and Additional Information
1. What is Soolantra and what is it used for
Soolantra contains the active ingredient ivermectin, which belongs to a group of medications called avermectins. The cream is applied to the skin of the face to treat pimples and inflammatory lesions that appear in rosacea.
Soolantra should only be used in adults (from 18 years of age).
2. What you need to know before using Soolantra
Do not use Soolantra:
- if you are allergic to ivermectin or any of the other ingredients of this medication (listed in section 6).
Warnings and Precautions
Consult your doctor or pharmacist before starting to use Soolantra.
At the start of treatment, some patients may experience a worsening of rosacea symptoms, however, this is rare and usually resolves within the first week of treatment. Talk to your doctor if this occurs.
Using Soolantra with Other Medications
Tell your doctor if you are taking, have recently taken, or may take any other medication, as these medications may affect your treatment with Soolantra.
Pregnancy and Breastfeeding
The use of Soolantra is not recommended during pregnancy.
If you are breastfeeding, you should not use this medication. Before starting treatment with Soolantra, you should stop breastfeeding.
You should consult your doctor to decide between using Soolantra and breastfeeding, considering the benefits of treatment with Soolantra or the benefits of breastfeeding.
Driving and Using Machines
The influence of Soolantra on the ability to drive and use machines is negligible.
Soolantra contains:
- Cetyl alcoholand stearyl alcoholwhich may cause local skin reactions (e.g., contact dermatitis).
- Methylparaben (E218)and Propylparaben (E216)which may cause allergic reactions (possibly delayed).
Propylene glycol which may cause skin irritation.
3. How to Use Soolantra
Follow your doctor's instructions for using this medication exactly. If in doubt, consult your doctor or pharmacist again.
Important:Soolantra is indicated for adults and should only be used on the skin of the face. Do not use this medication on any other part of your body, especially on moist body surfaces, e.g., eyes, mouth, or any mucous membrane. Do not ingest.
It is recommended to apply Soolantra to the face only once a day. You should apply a small amount of cream, the size of a pea, to each of the five areas of the face: forehead, chin, nose, and both cheeks, and then spread a thin, even layer of cream over each area.
You should avoid the eyelids, lips, and any mucous membranes, such as the inside of the nostrils, mouth, and eyes. If you accidentally apply the cream to the eyes or near the eyes, eyelids, lips, mouth, or mucous membranes, rinse the area immediately with plenty of water.
Do not apply any other cosmetics (such as other facial creams or makeup) before the daily application of Soolantra.
You should use these products only after applying the cream, once it has dried.
You should wash your hands immediately after applying the cream.
You should use Soolantra daily during the course of treatment, and the treatment course may be repeated. Your doctor will tell you how long you need to use Soolantra. The duration of treatment may vary from person to person, depending on the severity of the skin condition.
You will notice an improvement after 4 weeks of treatment. If there is no improvement after 3 months, you should stop treatment with Soolantra and consult your doctor.
Liver Impairment
If you have liver problems, please consult your doctor before using Soolantra.
Use in Children and Adolescents
Do not use Soolantra in children or adolescents.
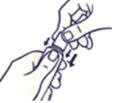
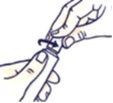
How to Open the Child-Resistant Tube
Do not squeeze the tube when opening or closing it to avoid spillage.
Press the cap and turn it counterclockwise (turn it to the left). Then remove the cap.
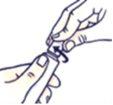
How to Close the Child-Resistant Tube
Press and turn it clockwise (turn it to the right).
If You Use More Soolantra Than You Should
If you use more than the recommended daily dose, contact your doctor, who will advise you on what action to take.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone: 91 562 04 20, indicating the medication and the amount ingested.
If You Forget to Use Soolantra
Do not apply a double dose to make up for the missed dose.
If You Stop Using Soolantra
The pimples and inflammatory lesions will decrease after several applications of this medication. It is important that you continue using Soolantra for the time your doctor has prescribed.
If you have any other questions about using this medication, ask your doctor or pharmacist.
4. Possible Side Effects
Like all medications, this medication can cause side effects, although not everyone will experience them. Soolantra may cause the following side effects:
Common side effects (may affect 1 to 10 people in 100):
- Burning sensation on the skin
Uncommon side effects (may affect 1 to 10 people in 1,000):
- Skin irritation - Itching of the skin - Dryness of the skin
- Worsening of rosacea (please consult your doctor)
Rare side effects (frequency cannot be estimated from the available data):
- Redness of the skin
- Inflammation of the skin
- Swelling of the face
- Increased liver enzymes (ALAT/ASAT)
Reporting Side Effects
If you experience any side effects, consult your doctor, even if they are not listed in this package leaflet. You can also report them directly through the Spanish Medicines and Health Products Agency's website: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medication.
5. Storage of Soolantra
Keep this medication out of the sight and reach of children.
Do not use this medication after the expiration date stated on the carton and tube. The expiration date is the last day of the month indicated.
Shelf life after first opening the tube: 6 months.
This medication does not require special storage conditions.
Medications should not be disposed of via wastewater or household waste. Return the containers and any unused medication to the pharmacy's SIGRE collection point. If in doubt, ask your pharmacist how to dispose of the containers and any unused medication. This will help protect the environment.
6. Package Contents and Additional Information
Composition of Soolantra
- The active ingredient is ivermectin. One gram of cream contains 10 mg of ivermectin.
- The other ingredients are glycerol, isopropyl palmitate, carbomer, dimethicone, disodium edetate, citric acid monohydrate, cetyl alcohol, stearyl alcohol, macrogol cetostearyl ether, sorbitan stearate, methylparaben (E218), propylparaben (E216), phenoxyethanol, propylene glycol, oleic acid, sodium hydroxide, purified water.
Appearance of Soolantra and Package Contents
Soolantra is a white to pale yellow cream. It is available in tubes of 2, 15, 30, 45, or 60 grams of cream. The larger tubes have a child-resistant closure, while the 2-gram tube does not.
Package with 1 tube.
Not all pack sizes may be marketed.
This medication is authorized in the Member States of the European Economic Area under the following names:
Austria, Germany, Portugal: | Soolantra 10 mg/g Cream |
Belgium, Luxembourg: | Soolantra 10 mg/g cream Soolantra 10 mg/g Creme |
Bulgaria: | Soolantra 10 mg/g ???? |
Cyprus, Greece: | Soolantra 10 mg/g Κρέμα |
Czech Republic, Hungary, Slovakia: Denmark: | Soolantra 10 mg/g krém Soolantra |
Estonia: | Soolantra 10 mg/g kreem |
Finland: | Soolantra 10 mg/g emulsiovoide |
France, Netherlands: | Soolantra 10 mg/g crème |
Iceland, Norway, Poland: | Soolantra 10 mg/g krem |
Ireland, United Kingdom: | Soolantra 10 mg/g cream |
Italy: | Efacti 10 mg/g Crema |
Latvia: | Soolantra 10 mg/g krems |
Lithuania: | Soolantra 10 mg/g kremas |
Malta: | Soolantra 10 mg/g krema |
Romania: | Soolantra 10 mg/g Crema |
Spain: | Soolantra 10 mg/g crema |
Sweden: | Soolantra 10 mg/g kräm |
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder:
Laboratorios Galderma, S.A.
Serrano Galvache, 56
28033 Madrid
Spain
Manufacturer:
Laboratoires Galderma
Z.I. – Montdésir
74 540 Alby-sur-Chéran
France
Date of the Last Revision of this Package Leaflet: February 2021
Detailed and updated information on this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.es
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to SOOLANTRA 10 mg/g CREAMDosage form: TABLET, 1 mgActive substance: finasterideManufacturer: Industrial Farmaceutica Cantabria S.A.Prescription requiredDosage form: TOPICAL SOLUTION, 2.275 mg/mlActive substance: finasterideManufacturer: Industrial Farmaceutica Cantabria S.A.Prescription requiredDosage form: TOPICAL SOLUTION, 50 mg/mlActive substance: minoxidilManufacturer: Mibe Pharma Espana S.L.Prescription not required
Online doctors for SOOLANTRA 10 mg/g CREAM
Discuss questions about SOOLANTRA 10 mg/g CREAM, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions








