
ALOCUTAN 50 mg/ml CUTANEOUS SPRAY SOLUTION

How to use ALOCUTAN 50 mg/ml CUTANEOUS SPRAY SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Leaflet: information for the patient
Alocutan50 mg/ml solution for cutaneous spray
Minoxidil
Read the entire leaflet carefully before starting to use this medication, as it contains important information for you.
Follow the administration instructions of the medication contained in this leaflet or as indicated by your doctor or pharmacist exactly.
- Keep this leaflet, as you may need to read it again.
- If you need advice or more information, consult your pharmacist.
- If you experience side effects, consult your doctor or pharmacist, even if they are side effects not listed in this leaflet. See section 4.
- You should consult a doctor if it worsens or does not improve after 4 months.
Contents of the leaflet
- What Alocutan 50 mg/ml is and what it is used for
- What you need to know before starting to use Alocutan 50 mg/ml
- How to use Alocutan 50 mg/ml
- Possible side effects
- Storage of Alocutan 50 mg/ml
- Contents of the pack and additional information
1. What Alocutan 50 mg/ml is and what it is used for
Alocutan 50 mg/ml prevents hereditary hair loss in men between 18 and 65 years old and stimulates hair growth. It also promotes the regeneration of new hair in already weakened or bald areas and thickens existing hair.
After application, Alocutan 50 mg/ml penetrates the scalp and reaches the hair roots. The mechanism by which hair growth is stimulated has not yet been clarified. However, the active principle of Alocutan 50 mg/ml may stop hereditary hair loss by:
- increasing the diameter of the hair shaft
- stimulating growth and
- prolonging the hair growth phases
However, at least four months will be needed before seeing any results. If the hair roots are still present, fine and weak hair may grow back, becoming longer, denser, and darker over time. The speed at which hair growth begins and how many hairs grow back depends on the patient. Large bald spots or hair loss present for more than 10 years are less sensitive to Alocutan 50 mg/ml.
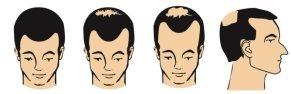
The following images show the stages of male pattern hair loss, for which the effectiveness of minoxidil, the active substance in Alocutan 50 mg/ml, has been demonstrated.
Children and adolescents under 18 years, patients 65 years and older
Alocutan 50 mg/ml should not be used in these patient groups, as there are no available efficacy and safety study results for these age groups.
2. What you need to know before starting to use Alocutan 50 mg/ml
Do not useAlocutan50 mg/ml
- if you are allergic to minoxidil or any of the other components of this medication (listed in section 6)
- if you are a woman, as there are occasional signs of cosmetically bothersome and reversible facial hair growth during treatment
- if you are using other medications on the scalp
- if you have any type of dressing or bandage on the scalp
- if you suffer from sudden or uneven hair loss
- if you have any condition that affects your scalp, including psoriasis (inflammatory skin condition with itching), sunburn, shaving of the scalp, or if the scalp skin is damaged by scars or burns, as there are no hair roots.
Warnings and precautions
Consult your doctor or pharmacist before using Alocutan 50 mg/ml.
Hormonal causes, underlying diseases, or malnutrition should be excluded. In these cases, specific treatment is needed.
Apply Alocutan 50 mg/ml only to healthy, normal scalp. You should not use Alocutan 50 mg/ml if the cause of hair loss is unknown or if hair loss is due to childbirth or if the scalp is reddened, inflamed, or painful.
There is no clinical experience to date regarding its effectiveness for hair loss in the temporal region (hairline recession).
If systemic effects (effects on internal organs) or severe skin reactions occur, treatment should be discontinued.
Alocutan 50 mg/ml is intended for external use only on the scalp. Do not apply Alocutan 50 mg/ml to other parts of the body.
You should not use Alocutan 50 mg/ml
- if you have any signs of cardiovascular disease or heart rhythm problems
- if you suffer from high blood pressure
- if you are taking medications to treat high blood pressure (antihypertensive agents).
You should stop using Alocutan 50 mg/ml and consult a doctor
- if you have low blood pressure or
- if one or more of the following symptoms appear: chest pain, faster heartbeats, weakness or dizziness, sudden and unexplained weight gain, swelling of hands or feet, persistent redness or irritation of the scalp or if other new, unexpected symptoms appear (see the section "If you use more Alocutan 50 mg/ml than you should").
Unwanted hair growth may be caused by transferring the product to areas other than the scalp.
Isolated cases of slight changes in hair color have been reported in patients with very blonde hair when using other hair care products at the same time or after swimming in highly chlorinated water.
Accidental ingestion can cause serious side effects in the cardiovascular system. Therefore, you should keep this medication out of the reach of children.
Cases of excessive body hair growth have been reported in infants after skin contact with the application areas of minoxidil in patients (caregivers) using topical minoxidil. Hair growth normalized within a few months when infants were no longer exposed to minoxidil. Precautions should be taken to ensure that children do not come into contact with the areas of the body where minoxidil has been applied topically.
Consult your doctor if you notice excessive hair growth on your child's body during the period when you are using topical minoxidil products.
Avoid inhaling the spray mist.
Children and adolescents
Do not administer this medication to children and adolescents under 18 years old, as safety and efficacy have not been established in this age group.
Other medications andAlocutan50 mg/ml
Tell your doctor or pharmacist if you are using/taking, have recently taken/used, or might take/use any other medication.
To date, there is no available information on interactions between Alocutan 50 mg/ml and other active substances. Although it is not clinically proven, there is a theoretical possibility that the absorption of the active substance in Alocutan 50 mg/ml (minoxidil) into the body may worsen orthostatic hypotension (a drop in blood pressure when standing up from a lying position) in patients who are also taking peripheral vasodilators (certain high blood pressure medications that widen blood vessels).
Alocutan 50 mg/ml should not be used with other dermatological products (preparations for external use containing active substances such as corticosteroids, retinoids, or ditranol) or with active substances that increase the absorption of the active substance through the skin (skin absorption).
Pregnancy and breastfeeding
Alocutan 50 mg/ml is indicated only for male patients.
Experience is limited with the application of Alocutan 50 mg/ml during pregnancy. Minoxidil absorbed by the body may pass into breast milk. Therefore, Alocutan 50 mg/ml should not be used by pregnant women and women during breastfeeding.
Driving and using machines
This product may cause dizziness or hypotension (see section 4). If you are affected, you should not drive or use machines.
Alocutan50 mg/ml contains propylene glycol (E 1520) and ethanol 96% (alcohol)
This medication contains 509 mg of propylene glycol (E 1520) in each ml of solution.
This medication contains 248 mg of alcohol (ethanol) in each ml of solution.
It may cause a burning sensation on damaged skin
Ethanol may cause itching and irritation in the eyes. In case of accidental contact with sensitive areas (eyes, skin abrasions, mucous membranes), rinse them with plenty of water.
If you repeatedly apply Alocutan 50 mg/ml to the hair instead of the scalp, this could increase dryness and/or stiffness of the hair due to the ethanol and propylene glycol content in Alocutan 50 mg/ml.
3. How to use Alocutan 50 mg/ml
Always use this medication exactly as described in this leaflet or as your doctor or pharmacist has told you. Consult your doctor or pharmacist if you are not sure.
Alocutan 50 mg/ml is for external use on the dry scalp. Use Alocutan 50 mg/ml only on healthy, undamaged scalp and follow the instructions for use exactly at all times. Do not apply Alocutan 50 mg/ml to areas of the body other than the scalp.
The recommended dose is:
Apply 1 ml of Alocutan 50 mg/ml twice a day (morning and evening) to the affected areas of the scalp.
Do not exceed the daily applied quantity, i.e., 2 x 1 ml solution, regardless of the size of the affected skin area.
Use in children and adolescents
Alocutan 50 mg/ml should not be used in children and adolescents under 18 years old, as there are no available safety and efficacy study results for these age groups.
Method of administration
Cutaneous use (scalp).
Each package of Alocutan 50 mg/ml contains 2 different spray applicators:
- pre-mounted applicator for large surface applications
- separate applicator with extended tip for smaller areas
Both applicators can be exchanged by separating the applicator and replacing it with the other.
For a 1 ml dose, 6 sprays are required.
Instructions for use/application:
The solution is sprayed directly onto the scalp within the hair loss area. To do this, press the pump down 6 times. After each pumping action, spread the liquid over the affected area with your fingertips. At the same time, avoid inhaling the spray mist.
What else you should consider during use
Your hands should be washed carefully after applying Alocutan 50 mg/ml to avoid accidental contact with the mucous membranes and eyes.
After applying Alocutan 50 mg/ml, you can comb your hair normally. However, you should not wet the scalp for approximately 4 hours. This will prevent Alocutan 50 mg/ml from being removed
Duration of treatment
The start and degree of hair growth are different in each patient.
In general, a twice-daily treatment for 2 to 4 months is required before an effect is observed. To maintain the effect, it is recommended to continue applying twice a day without interruption.
You will not get better results by applying Alocutan 50 mg/ml in larger quantities or more frequently. With regard to a possible therapeutic effect, there is sufficient clinical experience for a treatment period of up to one year.
If the desired therapeutic response is not obtained within 4 months, treatment should be discontinued.
Information on increased hair loss
In the treatment of hair follicles with the active substance minoxidil, the resting phase of the hair cycle (telogen phase) is shortened, and the growth phase (anagen phase) is reached more quickly. This stimulates the growth of new hair, which pushes the "old", no longer active hair out of the scalp. This gives the initial impression of increased hair loss. In some patients, this reaction has been observed 2 to 6 weeks after starting treatment with the active substance minoxidil. However, there is no need to be alarmed, as this reaction is accompanied by an increase in hair growth. The effect disappears within a few weeks and can be interpreted as a first sign of the effect of minoxidil.
Tell your doctor or pharmacist if you think the effect of Alocutan 50 mg/ml is too strong or too weak
If you use more Alocutan50 mg/ml than you should
Applying Alocutan 50 mg/ml at a dose higher than recommended and on relatively large body surfaces or areas other than the scalp may possibly lead to increased absorption of minoxidil into the body. To date, no cases are known in which the external use of the minoxidil solution has caused symptoms of intoxication.
After accidental ingestion, the concentration of the active component minoxidil in Alocutan 50 mg/ml may produce effects on internal organs that correspond to the effects that result when the active substance is ingested, for example, in a tablet. This may cause the following side effects: faster heartbeat, decreased blood pressure, fluid accumulation, and subsequent sudden weight gain, dizziness.
In case of accidental ingestion or if signs of overdose appear, inform your doctor immediately, so that he or she can decide what to do next. Please have the medication leaflet at hand so that the doctor can inform himself about which active substance has been taken.
If you forget to use Alocutan50 mg/ml
Do not apply a double dose to make up for a forgotten dose; continue your treatment at the recommended dose. Recovering the missed dose will not bring any benefit and could cause unwanted effects.
If you stop treatment with Alocutan50 mg/ml
Treatment needs to be continued to improve and maintain hair growth. Otherwise, hair loss will recur.
If treatment is stopped within 3 to 4 months, the effects correspond to those that would have been achieved without treatment with Alocutan 50 mg/ml.
If you have any further questions on the use of this medication, ask your doctor or pharmacist.
4. Possible side effects
Like all medications, this medication can cause side effects, although not everybody gets them.
Contact a doctor immediately if you notice any of the following symptoms: you may need urgent medical treatment.
- Swelling of the face, lips, or throat that makes swallowing or breathing difficult. This could be a sign of a severe allergic reaction (frequency unknown, cannot be estimated from available data)
- Generalized redness of the skin (frequency unknown, cannot be estimated from available data)
- Generalized itching (frequency unknown, cannot be estimated from available data)
- Throat tightness (frequency unknown, cannot be estimated from available data)
Very common(may affect more than 1 in 10 people):
- Headache
Common(may affect up to 1 in 10 people):
- Itching
- Increased hair growth beyond the scalp (including facial hair growth in women), inflammatory skin reaction (including acne-like rash, skin rash)
- Difficulty breathing, shortness of breath
- Swelling of arms and legs
- Weight gain
- High blood pressure
- Irritation of the scalp such as tingling, burning, dryness, itching, flaking, folliculitis
Uncommon(may affect up to 1 in 100 people):
- Dizziness, nausea
Unknown(frequency cannot be estimated from available data):
- Contact dermatitis (inflammation of the skin related to allergy)
- Depressed mood
- Eye irritation
- Faster heartbeat, palpitations, low blood pressure
- Vomiting
- Symptoms at the administration site that can also affect the ears and face, such as itching, skin irritation, pain, redness, swelling, dry skin, and inflammatory rash up to possible flaking of the skin, skin inflammation (dermatitis), blisters, bleeding, and ulceration
- Temporary hair loss, changes in hair color, altered hair structure
- Chest pain
Reporting of side effects
If you experience any side effects, consult your doctor or pharmacist, even if they are side effects not listed in this leaflet. You can also report them directly through the national reporting system included in the Spanish Pharmacovigilance System for Human Use Medicines: www.notificaram.es. By reporting side effects, you can contribute to providing more information on the safety of this medication.
5. Storage of Alocutan 50 mg/ml
Keep this medication out of the sight and reach of children.
Do not use this medication after the expiration date stated on the packaging and bottle after "EXP". The expiration date refers to the last day of that month.
Do not freeze.
Contains ethanol, which is flammable. Store away from heat sources or flames.
Note on expiration after opening
After opening the bottle, use the solution within 6 weeks.
Medications should not be disposed of via wastewater or household waste. Deposit the packaging and medications you no longer need at the pharmacy's SIGRE point. If in doubt, ask your pharmacist how to dispose of the packaging and medications you no longer need. This will help protect the environment.
6. Container contents and additional information
Composition ofAlocutan50 mg/ml
The active ingredient is minoxidil.
1 ml of solution contains 50 mg of minoxidil.
The other components are: ethanol 96% (v/v), propylene glycol (E1520), purified water.
Appearance ofAlocutan 50mg/ml and container contents
Alocutan 50 mg/ml is a clear, colorless to pale yellow solution and is available in 60 ml and 3 x 60 ml containers of cutaneous solution in white HDPE bottles.
Each container contains 2 different applicators, a pre-assembled pump spray applicator and an applicator with an extended tip.
Marketing authorization holder and manufacturer
Marketing authorization holder
Mibe Pharma España S.L.U.
C/Amaltea 9, 4th floor, letter B,
28045, Madrid
Spain
Manufacturer
Mibe GmbH Arzneimittel
Münchener Strasse, 15
06796 Brehna
Germany
This medicinal product is authorized in the EEA Member States under the following names:
Austria: Minoxidil Dermapharm 50 mg/ml Spray zur Anwendung auf der Haut, Lösung
Italy: Phalanx 50 mg/ml spray cutaneo, soluzione
Spain: Alocutan 50 mg/ml solution for cutaneous spray
Date of the last revision of this leaflet:October 2024
Detailed information on this medicinal product is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) (http://www.aemps.gob.es/).
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ALOCUTAN 50 mg/ml CUTANEOUS SPRAY SOLUTIONDosage form: TOPICAL SOLUTION, 5%Active substance: minoxidilManufacturer: Laboratorios Vinas S.A.Prescription not requiredDosage form: TOPICAL SOLUTION, 50 mg/mlActive substance: minoxidilManufacturer: Laboratorios Serra Pamies S.A.Prescription not requiredDosage form: TOPICAL SOLUTION, 2000 mgActive substance: minoxidilManufacturer: Laboratorios Galderma S.A.Prescription not required
Online doctors for ALOCUTAN 50 mg/ml CUTANEOUS SPRAY SOLUTION
Discuss questions about ALOCUTAN 50 mg/ml CUTANEOUS SPRAY SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions








