
SMOFKABIVEN NUTRIBASE EMULSION FOR INFUSION

How to use SMOFKABIVEN NUTRIBASE EMULSION FOR INFUSION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
SmofKabiven Nutribase Emulsion for Infusion
Read the entire package leaflet carefully before starting to use the medicine, as it contains important information for you.
- Keep this package leaflet, as you may need to read it again.
- If you have any questions, ask your doctor, pharmacist, or nurse.
- If you experience side effects, ask your doctor, pharmacist, or nurse, even if they are not listed in this package leaflet. See section 4.
Contents of the Package Leaflet:
- What SmofKabiven Nutribase is and what it is used for
- What you need to know before using SmofKabiven Nutribase
- How to use SmofKabiven Nutribase
- Possible side effects
- Storage of SmofKabiven Nutribase
- Contents of the pack and further information
1. What SmofKabiven Nutribase is and what it is used for
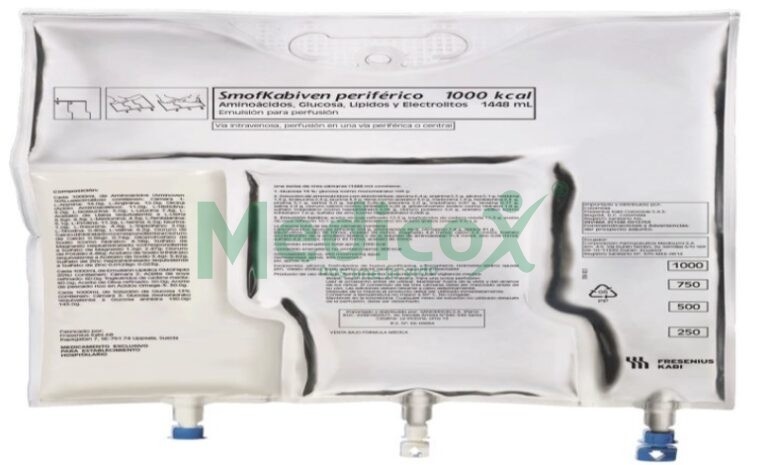
SmofKabiven Nutribase is an emulsion for infusion that is administered into your blood through a drip (intravenous infusion). The product contains amino acids (components used in the formation of proteins), glucose (carbohydrates), lipids (fat), and salts (electrolytes), in a plastic bag, and can be administered to adults and children from 2 years of age.
A healthcare professional will administer SmofKabiven Nutribase when other forms of nutrition are not sufficient or not possible.
2. What you need to know before using SmofKabiven Nutribase
Do not use SmofKabiven Nutribase:
- if you are allergic (hypersensitive) to the active substances or to any of the other components of this medicine (listed in section 6)
- if you are allergic to fish or eggs
- if you are allergic to peanuts or soy, you should not use this product. SmofKabiven Nutribase contains soybean oil
- if you have too much fat in your blood (hyperlipidemia)
- if you have severe liver damage
- if you have severe blood coagulation problems (severe coagulation disorders)
- if your body has congenital problems with the use of amino acids
- if you have severe kidney disease without the possibility of dialysis or hemofiltration
- if you are in acute shock (severe circulatory disorder)
- if you have too much sugar in your blood (hyperglycemia) that is not controlled
- if you have high levels of salts (electrolytes) in your blood that are found in SmofKabiven Nutribase
- if you have fluid in your lungs (acute pulmonary edema)
- if you have too much fluid in your body (overhydration)
- if you have heart failure that is not being treated
- if you have a defect in your blood coagulation system (hemophagocytic syndrome)
- if you are in an unstable situation, such as after severe trauma, uncontrolled diabetes mellitus, acute heart attack, stroke, blood clot, metabolic acidosis (a disorder that leads to too much acid in your blood), severe infection (sepsis), coma, and if you do not have enough fluid in your body (hypotonic dehydration)
- in newborns and children under 2 years of age
Warnings and precautions
Consult your doctor before starting to use SmofKabiven Nutribase if you have:
- kidney problems
- diabetes mellitus
- pancreatitis (inflammation of the pancreas)
- liver problems
- hypothyroidism (thyroid problems)
- sepsis (severe infection)
If during the infusion you experience fever, rash, swelling, difficulty breathing, chills, sweating, nausea, or vomiting, inform your healthcare professional immediately, as these symptoms may be caused by an allergic reaction or because you are receiving too much of the medicine.
It is possible that your doctor will need to regularly analyze your blood to perform liver function tests and other levels.
Children and adolescents
SmofKabiven Nutribase is not intended for newborns or children under 2 years of age.
SmofKabiven Nutribase can be administered to children from 2 to 18 years of age.
Other medicines and SmofKabiven Nutribase
Tell your doctor if you are taking, have recently taken, or may take any other medicine.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor before using this medicine.
There is no information on the use of SmofKabiven Nutribase during pregnancy. Therefore, SmofKabiven Nutribase should only be administered to pregnant women if the doctor considers it necessary. However, the use of SmofKabiven Nutribase may be considered during pregnancy if your doctor advises it.
No data are available on exposure in breastfeeding women.
The components and metabolites of parenteral nutrition such as SmofKabiven Nutribase are excreted in breast milk. Parenteral nutrition may be necessary during breastfeeding. SmofKabiven Nutribase should only be administered to breastfeeding women after their doctor has considered the potential risks and benefits.
Driving and using machines:
This is not relevant, as this medicine is administered in the hospital.
3. How to use SmofKabiven Nutribase
Follow exactly the administration instructions of this medicine indicated by your doctor. In case of doubt, consult your doctor again.
Your doctor will decide the dose for you individually, depending on your body weight and situation. SmofKabiven Nutribase will be administered to you by a healthcare professional.
If you use more SmofKabiven Nutribase than you should
It is very unlikely that you will receive too much of the medicine, as SmofKabiven Nutribase will be administered by a healthcare professional. In case of overdose or accidental ingestion, go to a medical center immediately or consult the Toxicology Information Service, phone: 91 5620420, indicating the medicine and the amount ingested.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Common(may affect up to 1 in 10 people): a slight increase in body temperature.
Uncommon(may affect up to 1 in 100 people): high levels of liver components in the blood, loss of appetite, nausea, vomiting, chills, dizziness, and headache.
Rare(may affect up to 1 in 1,000 people): low or high blood pressure, difficulty breathing, rapid heart rate (tachycardia). Hypersensitivity reactions (which can cause symptoms such as swelling, fever, low blood pressure, skin rash, hives, redness, headache). Feelings of cold and heat. Paleness. Blue discoloration of the lips and skin (due to lack of oxygen in the blood). Pain in the neck, back, bones, chest, and lumbar region.
Reporting of side effects
If you experience any side effect, tell your doctor or pharmacist, even if it is not listed in this package leaflet. You can also report side effects directly through the Spanish Pharmacovigilance System for Human Use: www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of SmofKabiven Nutribase
Keep this medicine out of the sight and reach of children.
Keep in the outer bag. Do not store above 25°C. Do not freeze.
Do not use this medicine after the expiry date which is stated on the label, on the bag, and on the carton. The expiry date is the last day of the month stated.
6. Container Content and Additional Information
Composition of SmofKabiven Nutribase
The active ingredients areg per 1,000 ml
Alanine | 4.7 4.7 |
Arginine | 4.1 |
Glycine | 3.7 |
Histidine | 1.0 |
Isoleucine | 1.7 |
Leucine | 2.5 |
Lysine (as acetate) | 2.2 |
Methionine | 1.5 |
Phenylalanine | 1.7 |
Proline | 3.8 |
Serine | 2.2 |
Taurine | 0.34 |
Threonine | 1.5 |
Tryptophan | 0.68 |
Tyrosine | 0.14 |
Valine | 2.1 |
Calcium chloride (as dihydrate) | 0.19 |
Sodium glycerophosphate (as hydrate) | 1.4 |
Magnesium sulfate (as heptahydrate) | 0.41 |
Potassium chloride | 1.5 |
Sodium acetate (as trihydrate) | 1.1 |
Zinc sulfate (as heptahydrate) | 0.0044 |
Glucose (as monohydrate) | 89 |
Soybean oil, refined | 12 |
Medium-chain triglycerides | 12 |
Olive oil, refined | 9.8 |
Fish oil, rich in omega-3 fatty acids | 5.9 |
The other components are: glycerol, purified egg phospholipids, all-rac-α-tocopherol, sodium hydroxide (pH adjustment), sodium oleate, acetic acid (pH adjustment), and water for injectable preparations.
Appearance of the Product and Container Content
SmofKabiven Nutribase, emulsion for infusion, consists of a three-chamber bag system where one chamber contains glucose solution, another contains amino acid solution, and another contains lipid emulsion. The glucose and amino acid solutions are transparent, colorless, or slightly yellow and free of particles. The lipid emulsion is white and homogeneous.
Container Sizes:
1 x 1,026 ml, 4 x 1,026 ml
1 x 1,539 ml, 4 x 1,539 ml
1 x 2,052 ml, 4 x 2,052 ml
1 x 2,565 ml, 3 x 2,565 ml
Only some container sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder:
Fresenius Kabi España, S.A.U.
Torre Mapfre – Vila Olímpica
Marina, 16-18
08005 Barcelona
Spain
Manufacturer
Fresenius Kabi AB,
Kraftvägen 1
SE-196 37 Kungsängen
Sweden
Date of Last Revision of this Leaflet:
November 2021
Detailed and updated information on this medicinal product is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.es/
This information is intended only for healthcare professionals (for more detailed information, please consult the technical sheet):
Special Warnings and Precautions for Use
To avoid the risks associated with too rapid infusion rates, it is recommended to use continuous and well-controlled infusion, if possible using an infusion pump.
Since the use of a central vein is associated with a high risk of infection, strict aseptic precautions should be taken to avoid any contamination during catheter insertion and handling.
Serum glucose, electrolytes, and osmolarity, as well as fluid balance, acid-base balance, and liver enzyme tests, should be monitored.
In the event of any signs or symptoms of anaphylactic reaction (such as fever, shivering, skin rash, or dyspnea), the infusion should be discontinued immediately.
SmofKabiven Nutribase should not be administered simultaneously with blood in the same infusion equipment due to the risk of pseudoagglutination.
Method of Administration
Intravenous route, infusion in a central vein.
To provide complete parenteral nutrition, trace elements, vitamins, and possibly electrolytes (taking into account the electrolytes already present in SmofKabiven Nutribase) should be added to SmofKabiven Nutribase, according to the patient's needs. The mixture within the SmofKabiven Nutribase bag should only be made when compatibility has been demonstrated, see the section on Special Precautions for Disposal and Other Handling.
Posology
Adults
Dosage:
The dose range of 18-40 ml SmofKabiven Nutribase/kg body weight/day will provide 0.10-0.22 g nitrogen/kg body weight/day (corresponding to 0.6-1.4 g amino acids/kg body weight/day) and 14-35 kcal/kg body weight/day of total energy (13-30 kcal/kg body weight/day of non-protein energy).
Infusion Rate:
The maximum infusion rate for glucose is 0.25 g/kg body weight/h, for amino acids 0.1 g/kg body weight/h, and for lipids 0.15 g/kg body weight/h.
The infusion rate should not exceed 2.8 ml/kg body weight/hour (corresponding to 0.25 g glucose, 0.09 g amino acids, and 0.11 g lipids/kg body weight/h). The recommended infusion period is 6.5-24 hours.
Maximum Daily Dose:
The maximum daily dose varies with the patient's clinical situation and may even change from day to day.
The recommended maximum daily dose is 40 ml/kg body weight/day.
Pediatric Population
Children (2-11 years)
Dosage:
The dose of up to 40 ml/kg body weight/day should be adjusted regularly according to the pediatric patient's requirements, which vary more than in adult patients.
Infusion Rate:
The maximum infusion rate is 3.4 ml/kg body weight/h (corresponding to 0.30 g glucose, 0.12 g amino acids, and 0.13 g lipids/kg body weight/h).
The recommended infusion period is 5-24 hours. At the maximum recommended infusion rate, do not use infusion periods greater than 11 hours and 45 minutes, except in exceptional cases and under close monitoring.
Maximum Daily Dose:
The maximum daily dose varies with the patient's clinical condition and may even change from day to day. The maximum daily dose is 40 ml/kg body weight/day.
Adolescents (12-18 years)
SmofKabiven Nutribase can be used in adolescents in the same way as in adults.
Precautions for Use
Do not use the container if it is damaged.
Use only if the amino acid and glucose solutions are transparent and colorless or slightly yellow, and if the lipid emulsion is white and homogeneous. The contents of the three separate chambers should be mixed before use and before making any additions through the additive port.
After opening the peel-type seals, the bag should be inverted three times to ensure a homogeneous mixture, which does not show evidence of phase separation.
For single use. Any remaining mixture after infusion should be discarded.
The disposal of unused medicinal products and all materials that have come into contact with them should be carried out in accordance with local regulations.
Compatibility
The compatibility table below shows the possible additions with the branded products Dipeptiven, Addaven, Vitalipid N Adult/Infant, and Soluvit N (lyophilized). The generated data support the additions to the activated bag according to the summary table below:
Total Maximum Content | |
Size of the SmofKabiven Nutribase bag | 1,026 ml, 1,539 ml, 2,052 ml, and 2,565 ml |
Additive | Volume |
Dipeptiven | 0 - 300 ml |
Addaven | 0 - 10 ml |
Soluvit N | 0 - 1 vial |
Vitalipid N Adult/Infant | 0 - 10 ml |
Note: This table is intended to present compatibility. It is not a dosing guide.
Additions should be made aseptically.
Validity Period after Mixing
The physical and chemical stability of the mixed three-chamber bag has been demonstrated for 36 hours at 25°C. From a microbiological point of view, the product should be used immediately. If not used immediately, the storage time before use and the storage conditions are the responsibility of the user and should normally not exceed 24 hours at 2-8 °C.
Validity Period after Mixing with Additives
From a microbiological point of view, the product should be used immediately after making the additions.
Instructions for Use of SmofKabiven Nutribase
The Bag
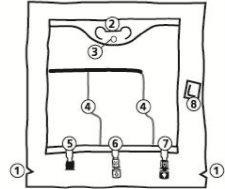
Notches on the overbag
Handle
Hole for hanging the bag
Breakable seals
Blind port (used only during manufacturing)
Additive port
Infusion port
Oxygen absorber
- Opening the overbag
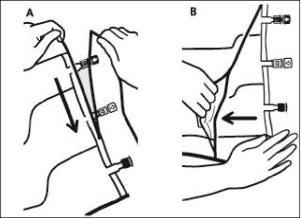
To remove the overbag, hold it in a horizontal position and tear along the notch towards the ports along the top edge (A)
Then, simply tear along the container; separate the overbag and discard it along with the oxygen absorber (B).
- Mixing
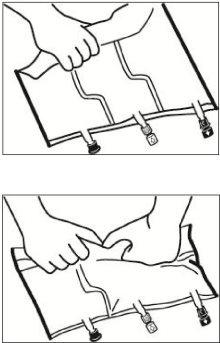
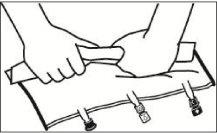
Place the bag on a flat surface.
Roll the bag from the hanger part towards the port part, first with the right hand and then applying constant pressure with the left hand until the vertical seals have opened. The vertical peel seals open due to the pressure of the liquid. The peel seals can also be opened before removing the overbag.
Note:The liquids mix easily, even if the horizontal seal remains closed.
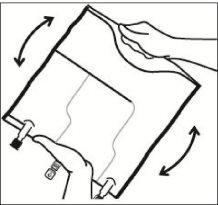
Mix the contents of the three chambers by inverting the bag three times until the components are completely mixed.
- Final Preparation:
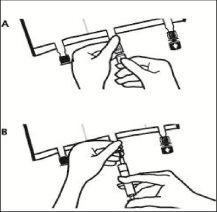
Place the bag back on a flat surface. Just before injecting the additives, break the white additive port at the arrow mark (A).
Note:The additive port membrane is sterile.
Hold the base of the additive port. Insert the needle, inject the additives (of known compatibility) through the center of the injection point (B).
Mix completely between each addition, inverting the bag three times. Use syringes with 18-23 gauge needles and a maximum length of 40 mm.
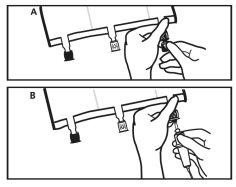
Just before inserting the infusion set, break the blue infusion port at the arrow mark (A).
Note:The infusion port membrane is sterile.
Use a non-vented infusion equipment or close the air inlet of the vented equipment.
Hold the base of the infusion port.
Insert the spike through the infusion port. The spike should be fully inserted to ensure its retention.
Note:The inside of the infusion port is sterile.
- Hanging the Bag
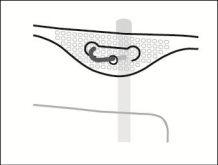
Hang the bag by the ring under the hanger.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to SMOFKABIVEN NUTRIBASE EMULSION FOR INFUSIONDosage form: INJECTABLE PERFUSION, 3.92 g / 1.26 g / 7.21 g / 3.36 g / 4.2 g / 5.11 g / 2.94 g / 2.8 g / 4.76 g / 5.07 g / 4.06 g / 14.49 g / 0.28 g / 8.05 g / 3.5 g / 200 gActive substance: combinationsManufacturer: Baxter S.L.Prescription requiredDosage form: INJECTABLE INFUSION, 3.5 g / 200 g / 5.22 g / 1.88 g / 3.92 g / 1.26 g / 7.21 g / 3.36 g / 4.2 g / 5.11 g / 2.94 g / 2.8 g / 662 mg / 1.02 g / 4.76 g / 5.15 g / 5.07 g / 4.06 g / 14.49 g / 0.28 g / 8.05 gActive substance: combinationsManufacturer: Baxter S.L.Prescription requiredDosage form: INJECTABLE PERFUSION, 4.25 g / 300 g / 5.22 g / 1.54 g / 4.76 g / 1.53 g / 8.76 g / 4.08 g / 5.1 g / 6.2 g / 3.57 g / 3.4 g / 662 mg / 1.02 g / 5.78 g / 5.94 g / 6.16 g / 4.93 g / 17.6 g / 0.34 g / 9.78 gActive substance: combinationsManufacturer: Baxter S.L.Prescription required
Online doctors for SMOFKABIVEN NUTRIBASE EMULSION FOR INFUSION
Discuss questions about SMOFKABIVEN NUTRIBASE EMULSION FOR INFUSION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















