
Smofkabiven Nutribase

Ask a doctor about a prescription for Smofkabiven Nutribase

How to use Smofkabiven Nutribase
Leaflet accompanying the packaging: information for the user
SmofKabiven Nutribase, infusion emulsion
Please read carefully the contents of the leaflet before using the medicine, as it contains
important information for the patient.
- Please keep this leaflet, so that you can read it again if you need to.
- In case of any doubts, please consult a doctor, pharmacist, or nurse.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should inform their doctor, pharmacist, or nurse. See section 4.
Table of contents of the leaflet
- 1. What SmofKabiven Nutribase is and what it is used for
- 2. Important information before using SmofKabiven Nutribase
- 3. How to use SmofKabiven Nutribase
- 4. Possible side effects
- 5. How to store SmofKabiven Nutribase
- 6. Contents of the packaging and other information
1. What SmofKabiven Nutribase is and what it is used for
SmofKabiven Nutribase is an infusion emulsion administered to the patient through a drip (intravenous infusion).
The packaging of the medicine consists of a plastic bag containing amino acids (components necessary for protein production), glucose (carbohydrates), fats (lipids), and salts (electrolytes). The medicine can be used in adult patients and children over 2 years of age.
SmofKabiven Nutribase is administered by medical professionals when other methods of nutrition are insufficient or impossible.
2. Important information before using SmofKabiven Nutribase
Do not use SmofKabiven Nutribase if the patient has:
- allergy to active substances or any of the other components of this medicine (listed in section 6);
- allergy to fish protein or eggs;
- allergy to peanuts or soy (SmofKabiven Nutribase contains soybean oil);
- too high a level of fats in the blood (hyperlipidemia);
- severe liver function disorders;
- severe blood coagulation disorders (severe coagulation disorders);
- inborn error of amino acid metabolism;
- severe kidney disease, without the possibility of dialysis or hemofiltration;
- acute shock (severe circulatory disorders);
- uncontrolled, increased blood glucose levels (hyperglycemia);
- increased levels of any of the electrolytes contained in SmofKabiven Nutribase in the blood (serum);
- fluid in the lungs (acute pulmonary edema);
- too much fluid in the body (overhydration);
- untreated heart failure;
- blood coagulation disorder (hemophagocytic syndrome);
- unstable general condition, e.g. severe post-traumatic condition, uncontrolled diabetes, acute myocardial infarction, stroke, thrombosis, metabolic acidosis (a condition characterized by too much acidic substance in the blood), severe infection (severe sepsis), coma, fluid deficiency (hypotonic dehydration).
SmofKabiven Nutribase should not be used in newborns and children under 2 years of age.
Warnings and precautions
Before starting treatment with the medicine, the patient should discuss with their doctor if they have:
- kidney disease;
- diabetes;
- pancreatitis;
- liver disease;
- hypothyroidism (thyroid disorders);
- sepsis (severe infection).
If during infusion the patient experiences fever, rash, swelling, difficulty breathing, chills, sweating, nausea, or vomiting, they should immediately inform the medical staff, as these symptoms may be caused by an allergic reaction or too high a dose of the medicine.
The doctor may recommend regular blood tests to determine liver function tests and other values.
Children and adolescents
SmofKabiven Nutribase is not intended for administration to newborns or children under 2 years of age. SmofKabiven Nutribase can be administered to children and adolescents from 2 to 18 years of age.
SmofKabiven Nutribase and other medicines
The patient should inform their doctor about all medicines they are currently taking or have recently taken, as well as any medicines they plan to take, including those available without a prescription.
Pregnancy and breastfeeding
If the patient is pregnant, thinks they may be pregnant, or plans to have a child, they should consult their doctor before using this medicine.
There is no data on the use of SmofKabiven Nutribase during pregnancy. SmofKabiven Nutribase is administered to pregnant women only when the doctor considers it necessary. SmofKabiven Nutribase may be administered during pregnancy on the doctor's instructions.
There is no available data on the use of SmofKabiven Nutribase in breastfeeding women.
Components and metabolites of parenteral nutrition, such as SmofKabiven Nutribase, pass into human milk. Parenteral nutrition may be necessary during breastfeeding. SmofKabiven Nutribase may be administered to breastfeeding women only after the doctor has considered the potential risks and benefits.
Driving and using machines
This does not apply, as SmofKabiven Nutribase is used in a hospital.
3. How to use SmofKabiven Nutribase
This medicine should always be used as directed by the doctor. In case of doubts, the patient should consult their doctor.
The doctor will choose an individual dose based on the patient's body weight and clinical condition. SmofKabiven Nutribase is administered by medical professionals.
Using a higher dose of SmofKabiven Nutribase than recommended
It is unlikely that the patient will receive too high a dose of SmofKabiven Nutribase, as the medicine is administered by medical professionals.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Common side effects(may occur in up to 1 in 10 patients):
slight increase in body temperature.
Uncommon side effects(may occur in up to 1 in 100 patients): high levels of liver enzymes in the blood, loss of appetite, nausea, vomiting, chills, dizziness, and headache.
Rare side effects(may occur in up to 1 in 1000 patients): low or high blood pressure, difficulty breathing, rapid heart rate (tachycardia).
Allergic reactions (which can cause symptoms such as swelling, fever, drop in blood pressure, skin rash, blisters, redness, headache). Feeling hot and cold. Paleness. Slight cyanosis of the lips and skin (related to hypoxia). Neck, back, bone, chest, and lumbar pain.
Reporting side effects
If the patient experiences any side effects, including any side effects not listed in this leaflet, they should inform their doctor, pharmacist, or nurse. Side effects can be reported directly to the Department of Adverse Reaction Monitoring of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, tel.: +48 22 49 21 301, fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl.
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help to gather more information on the safety of the medicine.
5. How to store SmofKabiven Nutribase
The medicine should be stored out of sight and reach of children.
Do not store above 25°C. Do not freeze. Store in the outer bag.
Do not use this medicine after the expiry date stated on the bag and the carton.
The expiry date refers to the last day of the month stated.
6. Contents of the packaging and other information
What SmofKabiven Nutribase contains
The active substances of the medicine are:
per 1000 ml
| alanine | 4.7 |
| arginine | 4.1 |
| glycine | 3.7 |
| histidine | 1.0 |
| isoleucine | 1.7 |
| leucine | 2.5 |
| lysine (as acetate) | 2.2 |
| methionine | 1.5 |
| phenylalanine | 1.7 |
| proline | 3.8 |
| serine | 2.2 |
| taurine | 0.34 |
| threonine | 1.5 |
| tryptophan | 0.68 |
| tyrosine | 0.14 |
| valine | 2.1 |
| calcium chloride (as dihydrate) | 0.19 |
| sodium glycerophosphate (as hydrated) | 1.4 |
| magnesium sulfate (as heptahydrate) | 0.41 |
| potassium chloride | 1.5 |
| sodium acetate (as trihydrate) | 1.1 |
| zinc sulfate (as heptahydrate) | 0.0044 |
| glucose (as monohydrate) | 89 |
| purified soybean oil | 12 |
| medium-chain triglycerides | 12 |
| purified olive oil | 9.8 |
| fish oil rich in omega-3 fatty acids | 5.9 |
The other ingredients (excipients) are: glycerol, purified egg phospholipids, all-rac-α-tocopherol, sodium hydroxide (for pH adjustment), sodium oleate, glacial acetic acid (for pH adjustment), and water for injections.
What SmofKabiven Nutribase looks like and what the pack contains
SmofKabiven Nutribase, infusion emulsion, is available in triple-chamber bags, where one chamber contains glucose solution, the second amino acids, and the third fat emulsion. The glucose and amino acid solutions are clear, colorless to slightly yellow, and free of particles. The fat emulsion is white and homogeneous.
Pack sizes:
1 x 1026 ml, 4 x 1026 ml
1 x 1539 ml, 4 x 1539 ml
1 x 2052 ml, 4 x 2052 ml
1 x 2565 ml, 3 x 2565 ml
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
Fresenius Kabi AB
Rapsgatan 7
751 74 Uppsala
Sweden
To obtain more detailed information, please contact the representative of the marketing authorization holder:
Fresenius Kabi Polska Sp. z o.o.
Al. Jerozolimskie 134
02-305 Warsaw
tel.: +48 22 345 67 89
This medicine is authorized in the Member States of the European Economic Area under the following names:
Austria
in progress
Belgium
SmofKabiven Nutribase emulsie voor infusie
SmofKabiven Nutribase émulsion pour perfusion
SmofKabiven Nutribase Emulsion zur Infusion
Bulgaria
СмофКабивен Централ Нутрибейз инфузионна емулсия
Croatia
SmofKabiven Nutribase emulzija za infuziju
Cyprus
SmofKabiven Nutribase
Denmark
SmofKabiven Nutribase
Estonia
SmofKabiven Nutribase
Finland
SmofKabiven Nutribase
France
SmofKabiven Nutribase émulsion pour perfusion
Greece
in progress
Spain
SmofKabiven Nutribase emulsión para perfusión
Netherlands
SmofKabiven Nutribase emulsie voor infusie
Ireland
SmofKabiven Nutribase
Iceland
SmofKabiven Nutribase innrennslislyf, fleyti
Lithuania
in progress
Luxembourg
in progress
Latvia
SmofKabiven Nutribase emulsija infūzijām
Germany
in progress
Norway
SmofKabiven Nutribase infusjonsvæske, emulsjon
Poland
SmofKabiven Nutribase
Portugal
SmofKabiven Nutribase
Czech Republic
SmofKabiven Nutribase
Romania
SmofKabiven Nutribase emulsie perfuzabilă
Slovakia
SmofKabiven Nutribase
Slovenia
SmofKabiven Nutribase emulzija za infundiranje
Sweden
SmofKabiven Nutribase
Hungary
SmofKabiven Nutribase emulziós infúzió
United Kingdom
SmofKabiven Nutribase
Date of last revision of the leaflet:
---------------------------------------------------------------------------------------------------------------------------
Information intended for healthcare professionals only (for further information, please refer to the Summary of Product Characteristics).
Warnings and precautions for use
In order to avoid the risks associated with infusion at a rate higher than recommended, it is recommended to perform it continuously and under proper control, if possible using a volumetric pump.
Since the use of a central vein for infusion is associated with an increased risk of infection, when inserting and handling the catheter, it is recommended to strictly follow the principles of aseptic procedure to avoid any infection.
It is recommended to monitor blood glucose and electrolyte levels, osmolality, and fluid and acid-base balance, as well as to perform liver enzyme tests.
In case of any signs or symptoms of anaphylactic reaction (such as fever, chills, rash, or difficulty breathing), the infusion should be stopped immediately.
SmofKabiven Nutribase should not be administered simultaneously with blood in the same infusion set, due to the risk of pseudoagglutination.
Method of administration
Intravenous administration, central vein infusion.
In order to ensure complete parenteral nutrition, it is recommended to add microelements, vitamins, and possibly electrolytes (taking into account the electrolytes already present in SmofKabiven Nutribase) to the medicine, according to the patient's needs. Mixing the components within the SmofKabiven Nutribase bag should only be done when their compatibility has been demonstrated, see section: Special precautions for disposal and preparation of the medicine for administration.
Any additives should be mixed with the medicine under aseptic conditions.
Dosage
Adult patients
Recommended dosage
The dose range is from 18 to 40 ml of SmofKabiven Nutribase/kg body weight/day, which provides 0.10 to 0.22 g of nitrogen/kg body weight/day (0.6 to 1.4 g of amino acids/kg body weight/day) and 16 to 35 kcal/kg body weight/day of total energy (13 to 30 kcal/kg body weight/day of non-protein energy).
Infusion rate
The maximum infusion rate of glucose is 0.25 g/kg body weight/hour, amino acids 0.1 g/kg body weight/hour, and fats 0.15 g/kg body weight/hour.
The infusion rate should not exceed 2.8 ml/kg body weight/hour (which corresponds to 0.25 g of glucose, 0.09 g of amino acids, and 0.11 g of fats/kg body weight/hour).
The recommended infusion time is from 6.5 to 24 hours.
Maximum daily dose
The maximum daily dose depends on the patient's clinical condition and may change even from day to day.
The recommended maximum daily dose is 40 ml/kg body weight/day.
Children and adolescents
Children from 2 to 11 years of age
Recommended dosage
The dose should be up to 40 ml/kg body weight/day and should be regularly adjusted according to the patient's needs, which may vary significantly more than in adult patients.
Infusion rate
The infusion rate should not exceed 3.4 ml/kg body weight/hour (which corresponds to 0.30 g of glucose/kg body weight/hour, 0.12 g of amino acids/kg body weight/hour, and 0.13 g of fats/kg body weight/hour).
The recommended infusion time is from 5 to 24 hours. Except in special situations and with careful monitoring, when using the recommended maximum infusion rate, the infusion time should not exceed 11 hours and 45 minutes.
Maximum daily dose
The maximum daily dose is variable depending on the patient's clinical condition and may change even from day to day. The recommended maximum daily dose is 40 ml/kg body weight/day.
Adolescents from 12 to 18 years of age
SmofKabiven Nutribase can be dosed in adolescents as in adult patients.
Special precautions for disposal and preparation of the medicine for administration
Do not use if the packaging is damaged.
Use only if the amino acid and glucose solutions are clear, colorless to slightly yellow, and the fat emulsion is white and homogeneous. The contents of the three separate chambers should be mixed before use and before adding any other substances through the dedicated port.
After removing the protective covers, roll the bag three times towards the ports to mix all the components of the medicine and obtain a homogeneous mixture, in which no signs of phase separation are visible.
For single use only. Any unused residue of the medicine remaining after infusion should be destroyed.
Any unused residue of the medicine or its waste should be disposed of in accordance with local regulations.
Compatibility
The following compatibility table shows the possibilities of adding Dipeptiven, Supliven, Vitalipid N Adult/Infant, and Soluvit N (lyophilized) to SmofKabiven Nutribase. Available data confirm the possibility of adding these medicines to the activated SmofKabiven Nutribase bag in accordance with the table below:
Note: this table is intended to show compatibility and does not provide dosage guidelines.
Any additives should be mixed with the medicine under aseptic conditions.
Shelf life after mixing
Physical and chemical stability of the mixed contents of the triple-chamber bag has been demonstrated for 36 hours at 25°C. From a microbiological point of view, the medicine should be used immediately. Otherwise, the user is responsible for the storage conditions during use and before administration. This period should not normally exceed 24 hours at 2-8°C.
| Maximum total content | |
| SmofKabiven Nutribase | 1026 ml, 1539 ml, 2052 ml, and 2565 ml |
| Added product | Volume |
| Dipeptiven |
|
| Supliven |
|
| Soluvit N |
|
| Vitalipid N Adult/Infant |
|
Shelf life after mixing with additional substances
From a microbiological point of view, the medicine should be used immediately after adding other components.
SmofKabiven Nutribase Instructions for preparing the bag for use
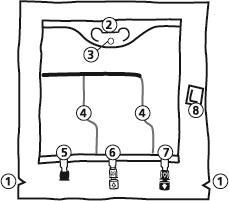
- 1. Notch in the outer bag
- 2. Bag handle
- 3. Hanging hole
- 4. Welds separating the individual chambers of the bag
- 5. Blind port (used only in production)
- 6. Port for adding substances
- 7. Infusion port
- 8. Oxygen absorber
1. Removing the outer bag
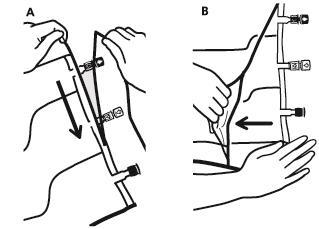
- To remove the outer bag, place it horizontally and tear it along the notch near the ports (A).
- Then tear the outer bag along the long edge, remove it, and discard it along with the oxygen absorber (B).
2. Mixing
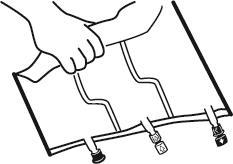
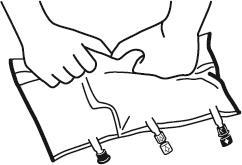
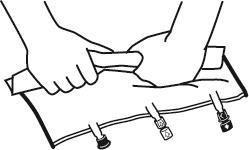
- Place the bag on a flat surface.
- Starting from the handle side, roll the bag firmly towards the ports, first with the right hand and then with the left hand, applying constant pressure, until the vertical welds open. They open under the pressure of the liquid. The welds can also be opened before removing the outer bag. Note: the liquid mixes easily, even though the horizontal weld remains intact.
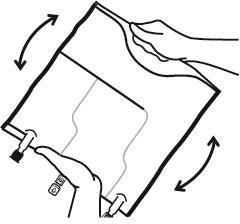
- Mix the contents of the three chambers by turning the bag three times, which should ensure thorough mixing of the components.
3. Final preparation steps
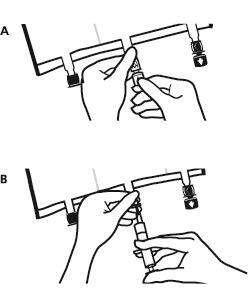
- Place the bag on a flat, even surface. Just before adding substances, remove the labeled plug from the white substance port (A). Note: the membrane of the substance port is sterile.
- Hold the base of the substance port. Introduce the needle and inject the substances (with known compatibility) through the center of the injection site (B).
- Mix the contents of the bag thoroughly after adding each component by turning the bag three times after each addition. Use syringes with needles with a diameter of 18 to 23 G and a maximum length of 40 mm.
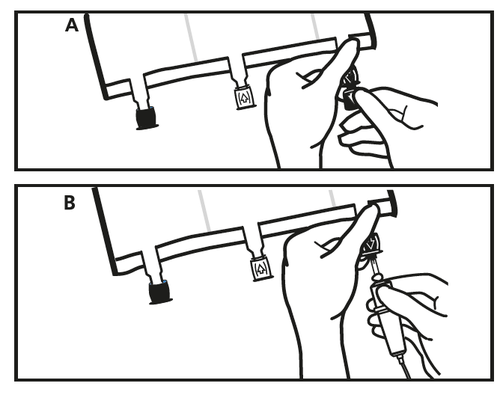
- Just before connecting the infusion set, remove the plug from the blue infusion port (A). Note: the membrane of the infusion port is sterile.
- Use infusion sets without an air vent or close the air vent.
- Hold the base of the infusion port.
- Introduce the needle of the infusion set into the infusion port. To ensure good fixation of the needle, insert its entire length. Note: the inner surface of the infusion port is sterile.
4. Hanging the bag
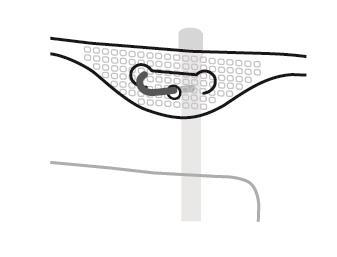
- Hang the bag using the hole located below the handle.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterFresenius Kabi AB
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Smofkabiven NutribaseDosage form: Solution, -Active substance: combinationsPrescription not requiredDosage form: Solution, -Active substance: combinationsPrescription not requiredDosage form: Solution, -Active substance: combinationsPrescription not required
Alternatives to Smofkabiven Nutribase in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Smofkabiven Nutribase in Spain
Alternative to Smofkabiven Nutribase in Ukraine
Online doctors for Smofkabiven Nutribase
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Smofkabiven Nutribase – subject to medical assessment and local rules.














