
Finomel Peri

Ask a doctor about a prescription for Finomel Peri

How to use Finomel Peri
Leaflet accompanying the packaging: Information for the user
Finomel Peri, infusion emulsion
You should carefully read the contents of the leaflet before using the medicine, as it contains
important information for the patient.
- You should keep this leaflet, so that you can read it again if you need to.
- If you have any doubts, you should consult a doctor or nurse.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor or nurse. See section 4.
Table of contents of the leaflet
- 1. What is Finomel Peri and what is it used for
- 2. Important information before using Finomel Peri
- 3. How to use Finomel Peri
- 4. Possible side effects
- 5. How to store Finomel Peri
- 6. Contents of the packaging and other information
1. What is Finomel Peri and what is it used for
Finomel Peri contains amino acids (components used to build proteins), glucose (carbohydrates), lipids (fats), and salts (electrolytes). Finomel Peri is used for nutrition in adults when normal oral nutrition is not sufficient or appropriate.
2. Important information before using Finomel Peri
When not to use Finomel Peri:
- if the patient is allergic to fish, eggs, soy, peanut proteins, or corn/corn products (see also "Warnings and precautions" below) or any of the other ingredients of this medicine (listed in section 6);
- if the patient has high blood fat levels;
- if the patient has severe liver disease;
- if the patient has blood coagulation disorders;
- if the patient has a disease involving the inability to process amino acids by the body;
- if the patient has severe kidney disease;
- if the patient has too high blood sugar levels;
- if the patient's blood has an abnormally high content of any of the electrolytes (sodium, potassium, magnesium, calcium, and/or phosphorus);
- if the patient has disorders during the administration of large volumes of fluids into the veins, such as acute pulmonary edema, overhydration, and uncontrolled heart failure;
- if the patient has any acute and severe health disorder, such as severe traumatic disorders, uncontrolled diabetes, acute myocardial infarction, stroke, embolism, metabolic acidosis, severe sepsis (bacteria in the blood), hypotonic dehydration, and hyperosmolar coma.
In each case, the doctor will decide on the administration of the medicine based on factors such as age, body weight, and the patient's clinical condition, including the results of tests performed.
Warnings and precautions
Before starting to use Finomel Peri, you should discuss it with your doctor or nurse if you have:
- severe kidney disease. You should also inform your doctor if you are undergoing dialysis (artificial kidney) or other blood purification methods;
- severe liver disease;
- blood coagulation disorders;
- adrenal insufficiency (adrenal gland dysfunction). The adrenal glands are triangular-shaped glands located on top of the kidneys;
- heart failure;
- lung disease;
- fluid accumulation in the body (overhydration);
- insufficient fluid in the body (dehydration);
- untreated high blood sugar levels (diabetes);
- heart attack or shock due to sudden heart failure;
- severe metabolic acidosis (too acidic blood);
- severe infection (sepsis).
If unusual signs or symptoms of an allergic reaction occur, such as fever, chills, skin rash, or difficulty breathing, the infusion should be stopped immediately. The medicine contains fish oil, soy oil, and egg yolk phospholipids, and glucose derived from corn, which may cause hypersensitivity reactions. Cross-allergic reactions have been observed between soy and peanut proteins. Difficulty breathing may also be a sign that small particles have formed, blocking blood vessels in the lungs (pulmonary embolism). If any breathing difficulties occur, you should tell your doctor or nurse. They will decide on the appropriate action.
Children and adolescents
There is no experience with the use of Finomel Peri in children and adolescents.
Finomel Peri and other medicines
You should tell your doctor or nurse about all medicines you are currently taking or have recently taken, as well as any medicines you plan to take. Finomel Peri contains calcium. It should not be administered together or through the same tube as the antibiotic ceftriaxone, as particles may form. If these medicines are administered sequentially using the same device, it should be thoroughly flushed. The olive oil and soy oil present in Finomel Peri contain vitamin K. This usually does not affect the action of blood-thinning medicines (anticoagulants) such as coumarin. However, if you are taking anticoagulant medicines, you should inform your doctor. The fats in the emulsion may interfere with the results of some laboratory tests if the blood sample for the test is taken before the fats are removed from the patient's bloodstream (they are removed from the blood after 5 to 6 hours after administration of fats).
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to have a child, you should consult your doctor before using this medicine. There is no data on the use of Finomel Peri during pregnancy and breastfeeding. The use of this medicine during pregnancy and breastfeeding may be considered if the doctor deems it necessary.
Driving and using machines
This is not relevant, as this medicine is administered in a hospital.
3. How to use Finomel Peri
This medicine should always be used as directed by your doctor. If you are unsure, you should consult your doctor. This medicine is administered as an intravenous infusion (drip) through a small tube directly into a vein. The doctor will determine the dose individually for each patient, depending on body weight and organ function. Finomel Peri will be administered by medical personnel.
Use in children
The safety and efficacy of this medicine have not been established in children and adolescents under 18 years of age.
Use of a higher than recommended dose of Finomel Peri
It is unlikely that a patient will receive too much of this medicine, as Finomel Peri is administered by medical personnel.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. The following side effects have been reported with an unknown frequency:
- hypersensitivity reactions (causing symptoms such as swelling, fever, low blood pressure, skin rashes, blisters (raised red areas), sudden skin redness, headache)
- re-feeding syndrome (a disease that occurs after receiving nutrition after prolonged starvation)
- high blood sugar levels (hyperglycemia)
- dizziness
- headache
- vein inflammation (thrombophlebitis)
- pulmonary embolism
- breathing difficulties
- nausea
- vomiting
- mildly elevated body temperature
- high blood levels (in serum) of liver-derived compounds
- fat overload syndrome
- infusion leak into surrounding tissues (extravasation)
Reporting side effects
If you experience any side effects, including any side effects not listed in the leaflet, you should tell your doctor or nurse. Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products Al. Jerozolimskie 181C PL 02-222 Warsaw Tel.: +48 22 49 21 301 Fax: +48 22 49 21 309 e-mail: [email protected] Side effects can also be reported to the marketing authorization holder. By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Finomel Peri
The medicine should be stored out of sight and reach of children. Store in protective bags. Do not freeze. Do not use this medicine after the expiry date stated on the label of the bag and on the carton. The expiry date refers to the last day of the month stated. Do not use this medicine if you notice visible particles in the solution or if the bag is damaged. Medicines should not be disposed of via wastewater or household waste. You should ask your pharmacist how to dispose of medicines that are no longer required. This will help protect the environment.
6. Contents of the packaging and other information
What Finomel Peri contains
- The active substances of the medicine are per 1000 mlAlanine 6.52 Arginine 3.62 Glycine 3.24 Histidine 1.51 Isoleucine 1.89 Leucine 2.30 Lysine (as lysine hydrochloride) 2.28 Methionine 1.26 Phenylalanine 1.76 Proline 2.14 Serine 1.58 Threonine 1.32 Tryptophan 0.57 Tyrosine 0.13 Valine 1.83 Sodium acetate trihydrate 1.77 Potassium chloride 1.41 Calcium chloride dihydrate 0.23 Magnesium sulfate heptahydrate 0.78 Sodium glycerophosphate hydrate 1.87 Zinc sulfate heptahydrate 0.007 Glucose (as glucose monohydrate) 77.8 Soybean oil 8.46 Olive oil 7.05 Triglycerides of saturated fatty acids of medium chain length 7.05
Fish oil rich in omega-3 fatty acids 5.64
- Other ingredients are: acetic acid, hydrochloric acid, egg phospholipids, glycerol, sodium oleate, all-rac-α-tocopherol, sodium hydroxide, water for injections.
What Finomel Peri looks like and what the pack contains
The glucose and amino acid solutions are clear and colorless to slightly yellow and do not contain solid particles. The fat emulsion is white and homogeneous. After mixing the contents of the 3 compartments, the medicine is a white emulsion. Packaging sizes: 4 x 1085 ml 4 x 1450 ml 4 x 2020 ml
Marketing authorization holder and importer
Marketing authorization holder: Baxter Polska Sp. z o.o. Ul. Kruczkowskiego 8 00-380 Warsaw Importer: Baxter SA Boulevard René Branquart 80 7860 Lessines Belgium
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
| Austria, Czech Republic, Germany, Greece, Ireland, Poland, Spain, United Kingdom | FINOMEL PERI |
| Belgium, Luxembourg, Netherlands | Periomegomel |
| Denmark, Finland, Iceland, Norway, Sweden | Finomel Perifer |
| France | FOSOMEL PERI |
| Italy | Finomel |
Date of last revision of the leaflet:
--------------------------------------------------------------------------------------------------------------------------------
Information intended for healthcare professionals only:
A. QUALITATIVE AND QUANTITATIVE COMPOSITION
Finomel Peri is packaged in triple-chamber, plastic bags. Each bag contains a sterile, apyrogenic 13% glucose solution, a 10% amino acid solution with electrolytes, and a 20% fat emulsion. The composition of the final emulsion after mixing the contents of the three chambers is given in the following table:
| Active substances | 1085 ml | 1450 ml | 2020 ml |
| Fish oil rich in omega-3 fatty acids | 6.12 g | 8.16 g | 11.40 g |
| Olive oil | 7.65 g | 10.20 g | 14.25 g |
| Soybean oil | 9.18 g | 12.24 g | 17.10 g |
| Triglycerides of saturated fatty acids of medium chain length | 7.65 g | 10.20 g | 14.25 g |
| Alanine | 7.08 g | 9.46 g | 13.17 g |
| Arginine | 3.93 g | 5.26 g | 7.31 g |
| Glycine | 3.52 g | 4.71 g | 6.55 g |
| Histidine | 1.64 g | 2.19 g | 3.05 g |
| Isoleucine | 2.05 g | 2.74 g | 3.82 g |
| Leucine | 2.50 g | 3.34 g | 4.64 g |
| Lysine (as lysine hydrochloride) | 1.98 g (2.48 g) | 2.65 g (3.31 g) | 3.69 g (4.61 g) |
| Methionine | 1.37 g | 1.83 g | 2.54 g |
| Phenylalanine | 1.92 g | 2.56 g | 3.56 g |
| Proline | 2.33 g | 3.11 g | 4.32 g |
| Serine | 1.71 g | 2.29 g | 3.18 g |
| Threonine | 1.44 g | 1.92 g | 2.67 g |
| Tryptophan | 0.62 g | 0.82 g | 1.14 g |
| Tyrosine | 0.14 g | 0.18 g | 0.25 g |
| Valine | 1.98 g | 2.65 g | 3.69 g |
| Sodium acetate trihydrate | 1.92 g | 2.57 g | 3.57 g |
| Potassium chloride | 1.53 g | 2.05 g | 2.85 g |
| Calcium chloride dihydrate | 0.25 g | 0.34 g | 0.47 g |
| Magnesium sulfate heptahydrate | 0.84 g | 1.13 g | 1.57 g |
| Sodium glycerophosphate hydrate | 2.03 g | 2.71 g | 3.77 g |
| Zinc sulfate heptahydrate | 0.008 g | 0.011 g | 0.015 g |
| Glucose (as glucose monohydrate) | 76.7 g (84.4 g) | 102.6 g (112.8 g) | 142.9 g (157.2 g) |
B. DOSAGE AND ADMINISTRATION
Dosage
Dosage should be individualized, depending on energy expenditure, the patient's clinical condition, body weight, and the ability to metabolize the components of Finomel Peri, as well as the energy and protein components additionally administered orally/enterally. Therefore, the size of the bag should be chosen accordingly. The average daily requirement for adult patients is: for patients with normal nutritional status or mild catabolic stress: 0.6 - 0.9 g amino acids/kg body weight/day (0.10 - 0.15 g nitrogen/kg body weight/day); for patients with moderate or high metabolic stress with or without malnutrition: 0.9 - 1.6 g amino acids/kg body weight/day (0.15 - 0.25 g nitrogen/kg body weight/day); for patients in special conditions (e.g., burns or significant anabolism), the nitrogen requirement may be even higher. The maximum daily dose varies depending on the patient's clinical condition and may change from day to day. The infusion rate should be increased gradually during the first hour. The infusion rate must be adjusted taking into account the administered dose, daily volume, and infusion duration. The recommended infusion duration is 14 to 24 hours. The dose range of 20 ml - 40 ml/kg body weight/day corresponds to 0.6 - 1.3 g amino acids/kg body weight/day (corresponding to 0.10 - 0.21 g nitrogen/kg body weight/day) and 14 - 27 kcal/kg body weight/day total energy (11 - 22 kcal/kg body weight/day non-protein energy). The maximum infusion rate of glucose is 0.25 g/kg body weight/hour, amino acids 0.1 g/kg body weight/hour, and fats 0.15 g/kg body weight/hour. The infusion rate should not exceed 3.0 ml/kg body weight/hour (corresponding to 0.09 g amino acids, 0.21 g glucose, and 0.09 g fats/kg body weight/hour). The recommended maximum daily dose is 40 ml/kg body weight/day and will provide 1.3 g amino acids/kg body weight/day (corresponding to 0.21 g nitrogen/kg body weight/day), 2.8 g glucose/kg body weight/day, 1.2 g lipids/kg body weight/day, and total energy of 27 kcal/kg body weight/day (corresponding to 22 kcal/kg body weight/day non-protein energy). Children and adolescents: No studies have been conducted with Finomel Peri in children and adolescents. Patients with renal/liver impairment: Caution should be exercised when administering to patients with liver dysfunction, including cholestasis and/or elevated liver enzymes. Liver function parameters should be closely monitored.
Administration
Intravenous administration, peripheral or central vein infusion. Instructions for reconstitution of the medicine before administration, see section E. Special precautions for disposal and preparation of the medicinal product for use. When using peripheral vein infusions, the osmolality of the solutions should be considered, as thrombophlebitis may occur. The infusion site should be evaluated daily for local signs of thrombophlebitis. Information on mixing with other infusion solutions/blood before or during administration, see section C. Incompatibilities.
C. INCOMPATIBILITIES
This medicine should not be mixed with other medicines with which its compatibility has not been established. This medicine should not be mixed or administered with ceftriaxone, a medicine containing calcium, including Finomel Peri. Finomel Peri should not be administered with blood through the same infusion set.
D. OVERDOSAGE
In case of overdose, nausea, vomiting, chills, hyperglycemia, and electrolyte disturbances, as well as signs of hypervolemia or acidosis, may occur. In such cases, the infusion must be stopped immediately. In case of hyperglycemia, treatment should be initiated according to the clinical situation, administering insulin and/or adjusting the infusion rate. Additionally, overdose may cause fluid overload, electrolyte disturbances, and hyperosmolality. If symptoms persist after stopping the infusion, hemodialysis, hemofiltration, or hemodiafiltration may be considered.
E. SPECIAL PRECAUTIONS FOR DISPOSAL AND PREPARATION OF THE MEDICINAL PRODUCT FOR USE
Opening:
- Remove the protective bag.
- Discard the oxygen absorber sachet.
- Use only if the bag is undamaged and the seals are intact (i.e., the contents of the three chambers have not been mixed), the amino acid and glucose solutions are clear, colorless, or slightly yellow, and free of visible particles, and the fat emulsion is homogeneous with a milky appearance.
Mixing the contents of the chambers:
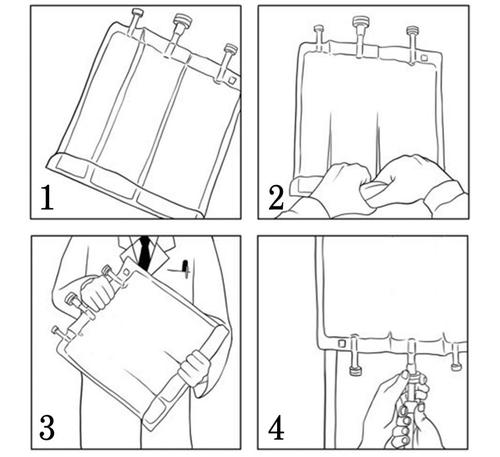
- Before breaking the seals, ensure that the medicine is at room temperature.
- Starting from the top of the bag (at the handle), roll the bag with both hands (Figure 1). The seals will break from the port side. Continue rolling the bag until the seals break halfway through their length. (Figure 2)
- Mix by turning the bag at least 3 times. (Figure 3)
- After mixing, the medicine is a homogeneous emulsion with a milky appearance.
After removing the protective cap from the medication port, additional components can be added through the medication port (see "Addition"). Remove the protective cap from the infusion port and connect the infusion set. Hang the bag on an infusion stand and perform the infusion using standard technique. (Figure 4)After opening the bag, the contents should be used immediately and the opened bag should not be stored for the next infusion. Do not reconnect partially used bags. To avoid the possibility of air embolism, do not connect bags in series.
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterBaxter S.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Finomel PeriDosage form: Solution, -Active substance: combinationsPrescription not requiredDosage form: Solution, -Active substance: combinationsPrescription not requiredDosage form: Solution, -Active substance: combinationsPrescription not required
Alternatives to Finomel Peri in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Finomel Peri in Spain
Alternative to Finomel Peri in Ukraine
Online doctors for Finomel Peri
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Finomel Peri – subject to medical assessment and local rules.














